Basic Neurophysiology and Neuroanatomy
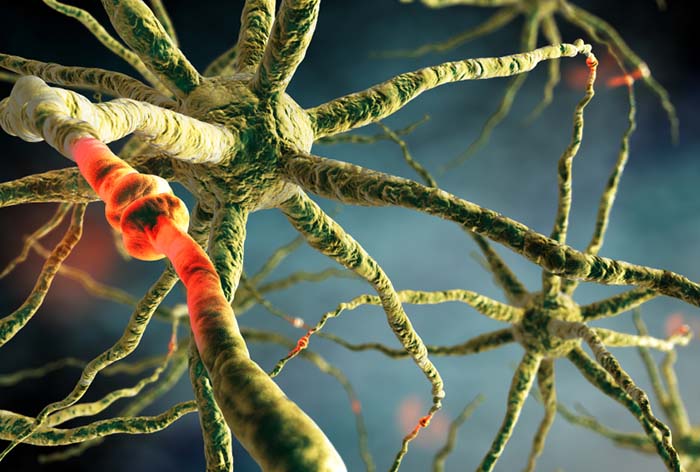
The brain uses a sophisticated communication and command-and-control system that monitors and manages interactions between roughly 100 billion neurons, each with 5,000-10,000 synaptic connections, for as many as 500 trillion synapses in adults (Breedlove & Watson, 2020).
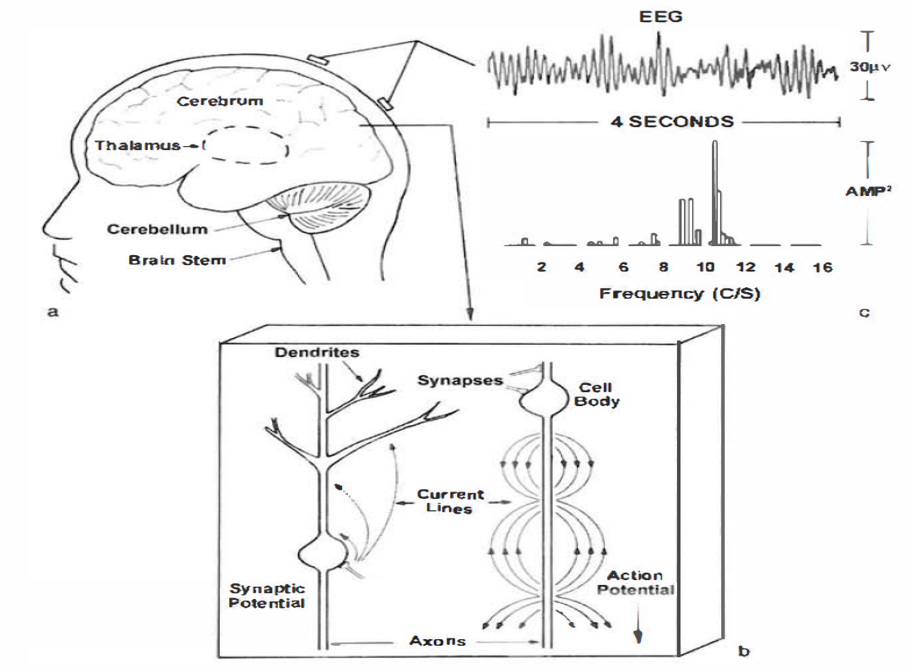
An electroencephalograph (EEG) monitors brainwave activity at various frequencies, from DC shifts (slow cortical potentials) to fast potentials exceeding 50 Hz. The EEG records the excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) propagated by the apical dendrites of large pyramidal cells arranged in thousands of cortical columns. Local field potentials, the aggregate effect of interconnected neuron firing and modulation by glial cells, regulate neuron excitability and firing. Action potential animation © NIMEDIA/Shutterstock.com.
Neuroplasticity, the remodeling of neurons and neural networks with experience, is responsible for learning and memory and makes neurofeedback training possible.
MCP Blueprint Coverage
This unit addresses II. Basic Neurophysiology and Neuroanatomy.
A. NEUROPHYSIOLOGY

This unit covers the Bioelectric Origin and Functional Correlates of EEG, Definition of ERPs and SCPs, and Neuroplasticity.
Bioelectric Origin and Functional Correlates of EEG
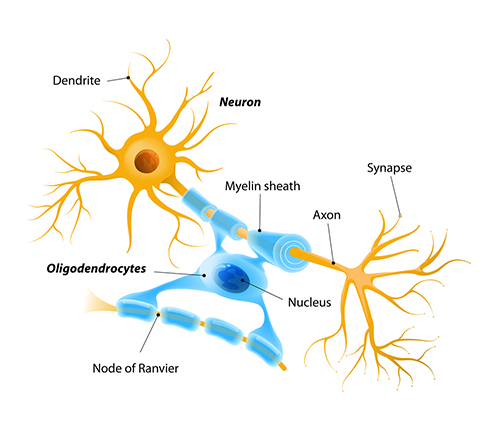
Types of Neurons
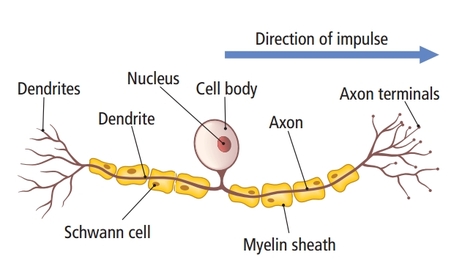
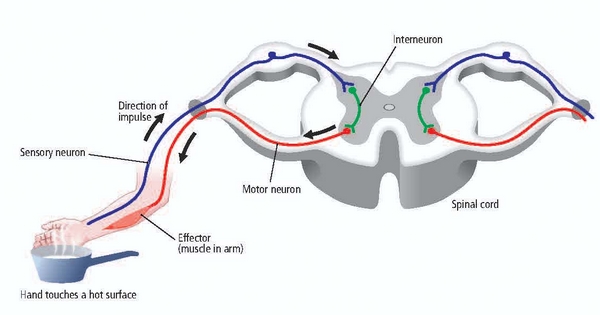
Sensory neurons are specialized for sensory intake. They are called afferent because they transmit sensory information towards the central nervous system (brain and spinal cord). The graphic below is courtesy of leavingcertbiology.net.
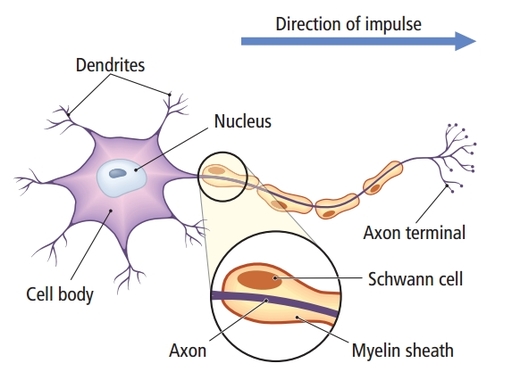
Motor neurons convey commands to glands, muscles, and other neurons. They are called efferent because they convey information towards the periphery. Graphic courtesy of leavingcertbiology.net.
Interneurons provide the integration required for decisions, learning and memory, perception, planning, and movement. They have short processes, analyze incoming information, and distribute their analysis with other neurons in their network. Interneurons are entirely confined to the central nervous system, account for many of its neurons, and comprise most of the brain (Breedlove & Watson, 2020). Local interneurons analyze small amounts of information provided by neighboring neurons. Relay interneurons connect networks of local interneurons from separate regions to enable diverse functions like perception, learning, and memory, and executive functions like planning (Carlson & Birkett, 2021). Graphic courtesy of leavingcertbiology.net.
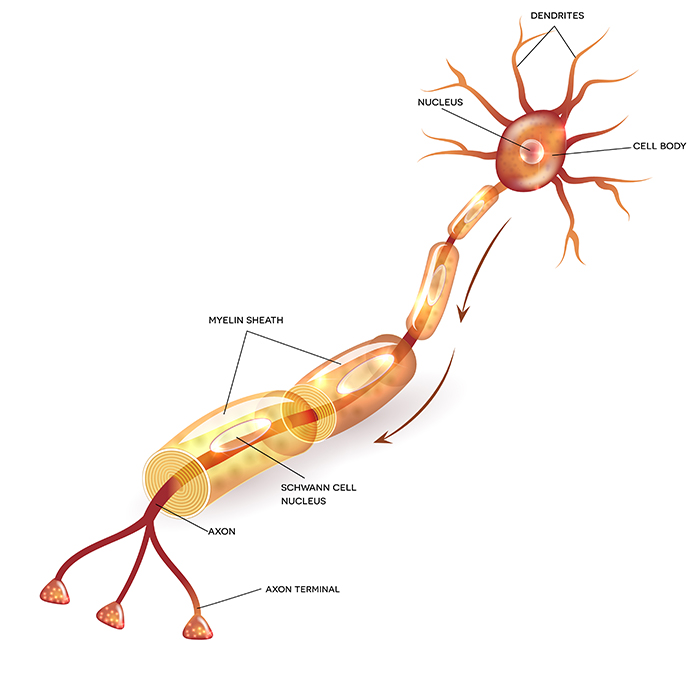
Neuron Structure
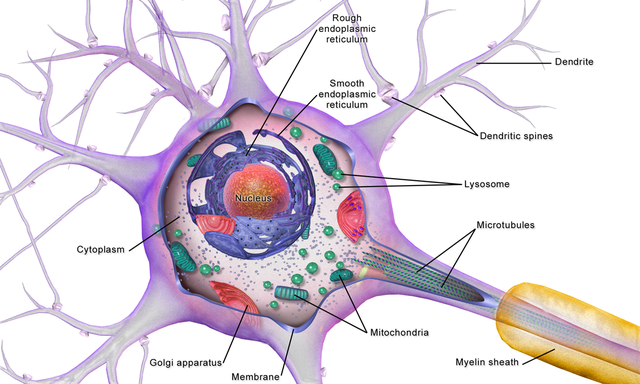
While neurons have over 200 different designs to perform specialized jobs in the nervous system, they generally have five structures: a cell body or soma, dendrites, an axon and axon hillock, and terminal buttons. Check out the Blausen Neuron Structure in CNS animation. Graphic © Designua/Shutterstock.com.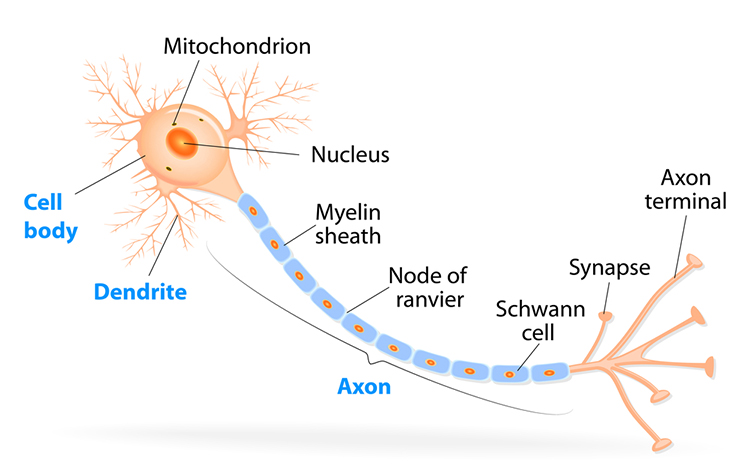
The
cell body or soma contains the machinery for the neuron’s
life processes. It receives and integrates EPSPs and IPSPs, small graded positive and negative changes in
membrane potential generated by axons. The cell body of a typical neuron is
20 μm in diameter, and its spherical nucleus,
which contains chromosomes comprised of DNA, is 5-10
μm across. The cell body is the only location where neurons manufacture proteins (like enzymes,
receptors, and ion channels) and peptides (neurotransmitters like oxytocin) since this requires ribosomes. Check out the Khan Academy YouTube video, Anatomy of a Neuron.
Dendrites are branched structures designed to receive messages from other neurons via axodendritic synapses (junctions between axons and dendrites shown below) and send messages to other neurons dendrodendritic synapses (junctions between the dendrites of two neurons). Dendrites receive thousands of synaptic contacts and have specialized proteins called receptors for neurotransmitters released into the synaptic cleft (Bear, Connors, & Paradiso, 2020).
A neuron's dendrites are called a dendritic tree, and each branch of the tree is called a dendritic branch. Graphic by BruceBlaus from Wikipedia article Neuron.
Biological psychologists classify neurons based on whether their dendrites feature spines. Dendritic spines are protrusions on the dendrite shaft where axons
typically form axodendritic synapses. Graphic © Jose Luis Calvo/Shutterstock.com.
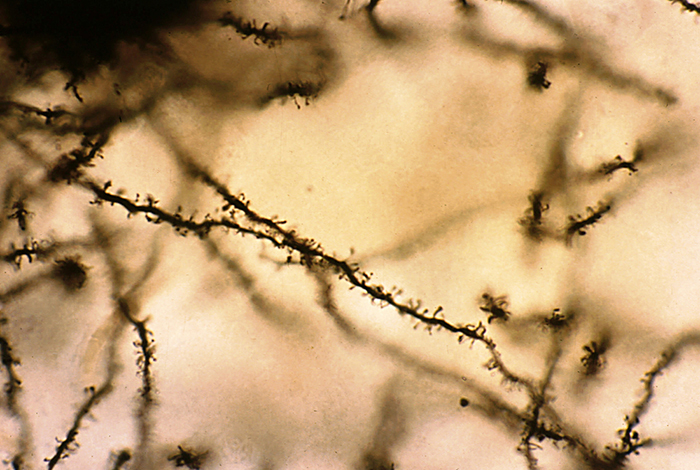
Spiny neurons have dendritic spines, while aspinous neurons do not (Bear, Connors, & Paradiso, 2020).
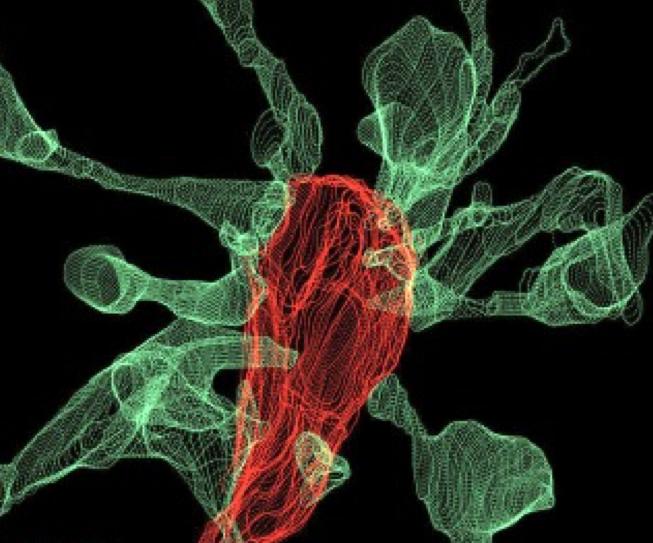
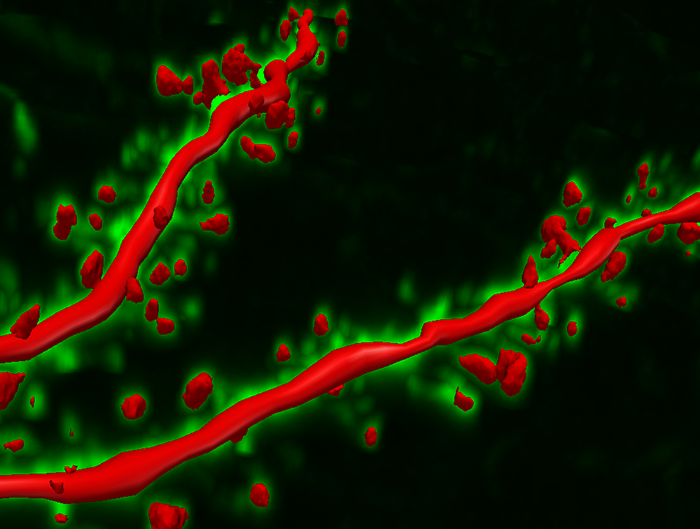
During learning, spines' number, size, and shape may change to adjust the space for receptors (neuroplasticity). Microglia shown in green participate in the remodeling process. Graphic © Genetic Engineering and Biotechnology News.
An axon is a cylindrical structure only found in neurons that is specialized for the distribution of information within the central and peripheral nervous systems. Axons range from 1 to 25 µm in diameter and 0.1 mm to more than a meter in
length. Over 90% of neurons are interneurons whose axons and dendrites are very short and do not
extend beyond their cell cluster. Axons usually branch repeatedly. Each branch is called an axon collateral.
Axons transmit
action potentials
toward a neuron's terminal buttons. Using microtubules, an axon also bidirectionally transports molecules between the cell body and terminal buttons.
An axon hillock is a swelling of the cell body where the axon
begins. The middle of an axon is the axon proper, and the end is the axon terminal (Bear, Connors, & Paradiso, 2020). Graphic by M.alijar3i from the Wikipedia article Axon Hillock.
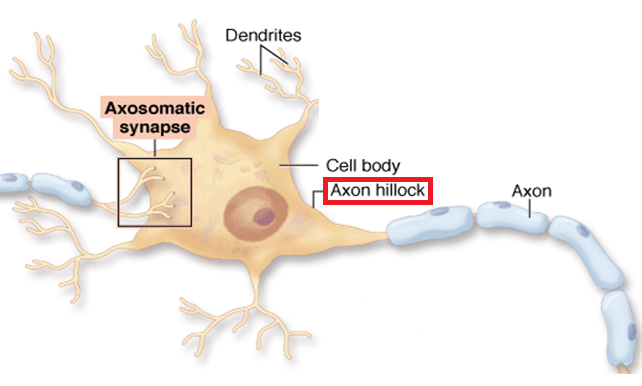
The axon hillock sums EPSPs and IPSPs over milliseconds to generate an action potential.
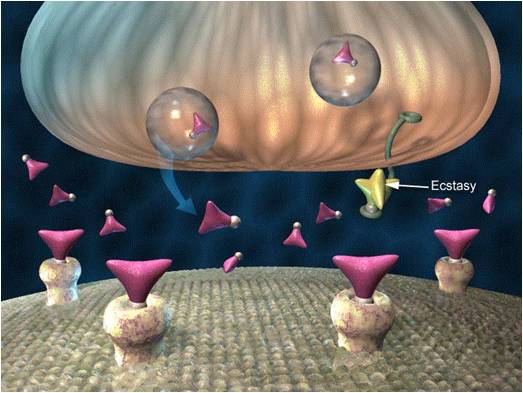
Axon terminals are buds located on the ends of axon branches
that form synapses and release neurochemicals to other neurons. Axon terminals contain vesicles that store
neurotransmitters for release when an action potential arrives. Their presynaptic membrane
may have reuptake transporters that return neurotransmitters from the synapse or extracellular space for
repackaging. The graphic of serotonin reuptake transporters below is courtesy of NIDA.
Types of Glial Cells
While there are hundreds of types of neurons, there are only four main categories of glial cells (astrocytes, microglia, oligodendrocytes, and Schwann cells).Old school view: glial cells mainly provide structural support (glia is derived from the Greek for glue).
New school view: glial cells help neurons process information, including modulating neuron excitability.
Check out the YouTube video, Neurology - Glial Cells, White Matter and Gray Matter.
Astrocytes (shown below) are star-shaped and are the most prevalent glial cells in the brain. They guide neuronal migration in the embryo and fetus. Since they occupy most of the expanse between neurons and are separated from neurons by nearly 20 µm, they may affect the growth or retraction of axons and dendrites (collectively called neurites). An emerging view is that astrocytes help neurons process information and communicate with each other through parallel astrocyte-astrocyte networks. Astrocyte membranes contain neurotransmitter receptors that can initiate changes in their membrane potential and internal biochemical processes.
Astrocytes regulate circulating molecules in the extracellular space (the region surrounding neurons) and the synapse. They enclose synapses, limit the movement of released neurotransmitters, and transport them from the synapse to the axon terminal. They also regulate the concentration of ions like potassium outside of neurons to prevent interference with their performance (Bear, Connors, & Paradiso, 2020).
Astrocytes transport nutrients, remove wastes, store glycogen during stage-3 sleep, dynamically control local blood flow, and develop and maintain the blood-brain barrier.
Astrocytes develop and maintain
the blood-brain barrier. Graphic © Designua/Shutterstock.com.
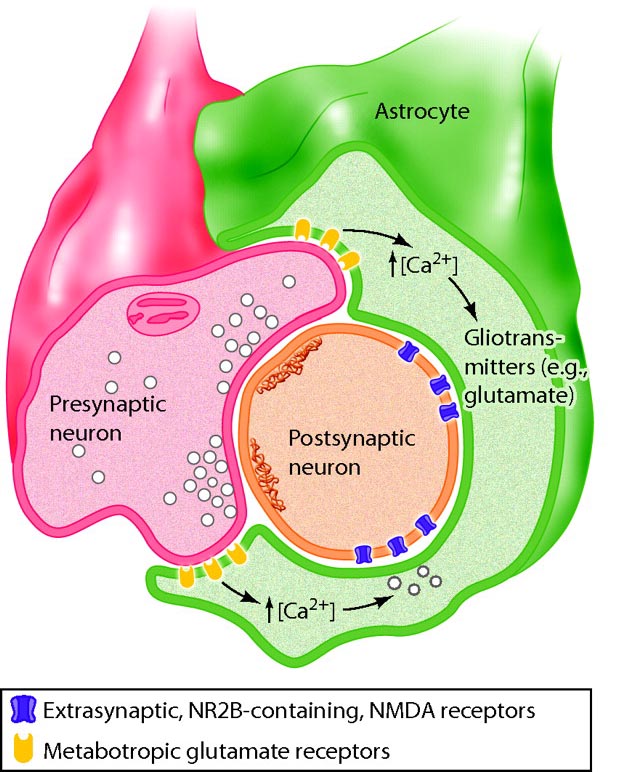
Astrocytes directly receive synapses from neurons and integrate neuronal messages, monitor the activity of
nearby synapses, communicate with each other using calcium ions and ATP, converse with nearby neurons using
neurotransmitters, and strongly influence the number of synapses. Schwann cells help determine synapse location.
Graphic © 2016 physiologyonline.physiology.org.
Microscopic microglial cells participate in the immune
response. Microglial cells scavenge and engulf diverse materials (phagocytosis), release cytotoxins to control infection,
present antigens to T-cells, remove branches from neurons near damaged tissue to aid regrowth (synaptic
stripping), promote tissue repair, and promote chronic neuroinflammation in the CNS that amplifies
neurodegeneration. They assist synaptic remodeling by removing unnecessary synapses. Finally, microglia cross the blood-brain barrier to promote homeostasis (Bear, Connors, & Paradiso, 2020). Graphic © Juan Gaertner/Shutterstock.com.
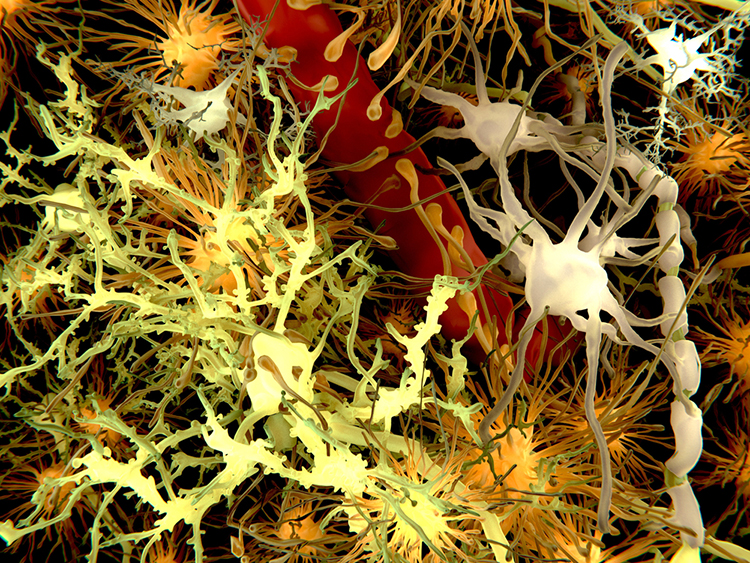
Description: yellow = neurons, orange = astrocytes, grey = oligodendrocytes, white = microglia.
Oligodendrocytes, which are smaller than astrocytes, form up to 50
segments of myelin that only insulate adjacent axons within the brain and spinal cord of the central nervous system. Check out the Blausen Neural Tissue: Oligodendrocyte animation. Graphic © Designua/Shutterstock.com.
Oligodendrocytes block axonal regeneration by releasing growth inhibitory proteins. These molecules are part of the reason
for minimal functional recovery in the CNS following spinal cord damage. Multiple
sclerosis, a demyelinating disease, destroys oligodendrocytes.
Check out the Blausen Demyelination in the CNS: Multiple Sclerosis animation.
Schwann cells provide myelin for single PNS axons and facilitate
axonal regeneration following damage (Breedlove & Watson, 2020). Check out the Blausen Neuroglia: Schwann Cell on Myelinated Peripheral Neuron animation.
Graphic © Tefi/Shutterstock.com.
Excitatory and Inhibitory Postsynaptic Potentials
Graded positive and negative changes in membrane potential, called excitatory postsynaptic potentials and inhibitory postsynaptic potentials, are essential to the EEG and communication among neurons.An excitatory postsynaptic potential (EPSP) is a subthreshold depolarization that makes the membrane potential more positive and pushes the neuron towards its excitation threshold. EPSPs are produced when neurotransmitters bind to receptors and cause positive sodium ions to enter the cell. At a single synapse, a postsynaptic membrane may have tens to thousands of transmitter-gated ion channels. The amount of transmitter released determines how many of these channels will be activated. The size of an EPSP will be a multiple of the number of vesicles, each containing several thousand transmitter molecules. Check out the Blausen Positive Potential animation.
An inhibitory postsynaptic potential (IPSP) is a hyperpolarization that makes the membrane potential more negative and pushes the neuron away from its excitation threshold. At most inhibitory synapses, IPSPs are produced when neurotransmitters like GABA or glycine bind to receptors and cause negative chloride ions to enter the cell. When an inhibitory synapse is closer to the soma than an excitatory synapse, it can counteract positive current flow and decrease the size of the EPSP. This mechanism is called shunting inhibition (Bear, Connors, & Paradiso, 2020).
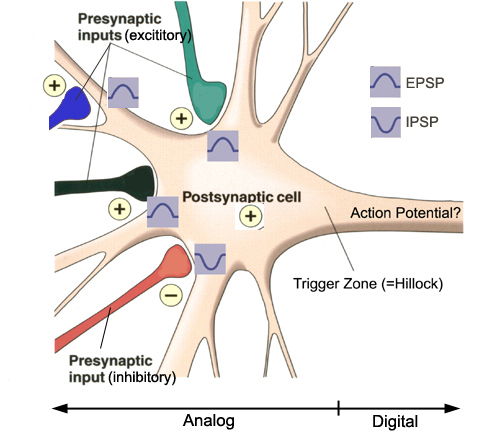
Integrating Postsynaptic Potentials
Integration is the summation of EPSPs and IPSPs at the unmyelinated axon hillock.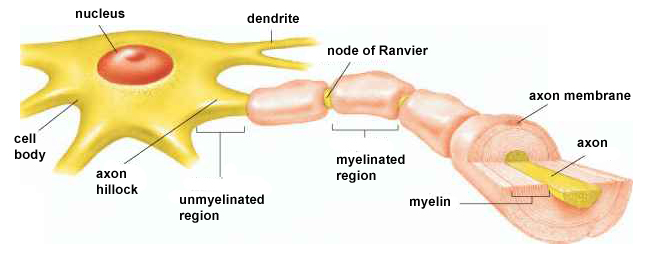
The axon hillock of a postsynaptic neuron uses two methods to sum EPSPs and IPSPs: spatial and temporal summation.
In spatial summation, the axon hillock sums the simultaneous postsynaptic potentials (PSPs) from thousands of synapses on dendrites. In temporal summation, the axon hillock adds the PSPs from presynaptic neurons that repeatedly fire within a 1-15-ms time window.
Each EPSP depolarizes the axon hillock by about 0.5 mV. If there were no competing IPSPs, it would take about 30 EPSPs to trigger an action potential. Each IPSP hyperpolarizes the axon hillock by about 0.5 mV. If the summated EPSPs and IPSPs move the axon hillock from a resting potential of -70 mV to a threshold of excitation of -55 mV, sodium channels in the axon hillock membrane open, and an action potential propagates down the axon. Graphic © 2003 Josephine Wilson.
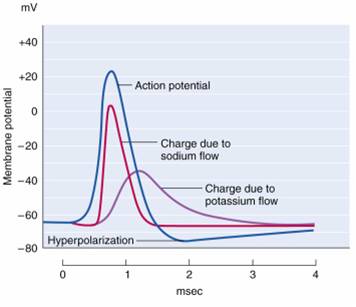
Check out the YouTube video, Best Action Potential Explanation.
Action Potentials
An action potential is a brief electrical impulse that transmits information from the axon hillock to the terminal button. This wave of positive charge only travels in one direction because the preceding segment is refractory due to the closing of its sodium channels. An action potential takes 1-2 ms from the point the axon hillock reaches its threshold to its repolarization to a negative resting potential.Watch the Blausen Action Potential animation.
Action potentials travel down axons, which branch multiple times and terminate at synapses. The all-or-none law and rate laws describe action potential transmission. The all-or-none law states that once an action potential is triggered in an axon, it is propagated, without decrement, to the end of the axon. The rate law states that neurons represent the intensity of a stimulus by variation in the rate of axon firing. More intense stimuli shorten the interval before a neuron can fire again, allowing a neuron to fire more rapidly. An intense stimulus can cause a neuron to fire every 2 or 3 ms, while a weak stimulus might lengthen the time lag to every 4 or 5 ms.
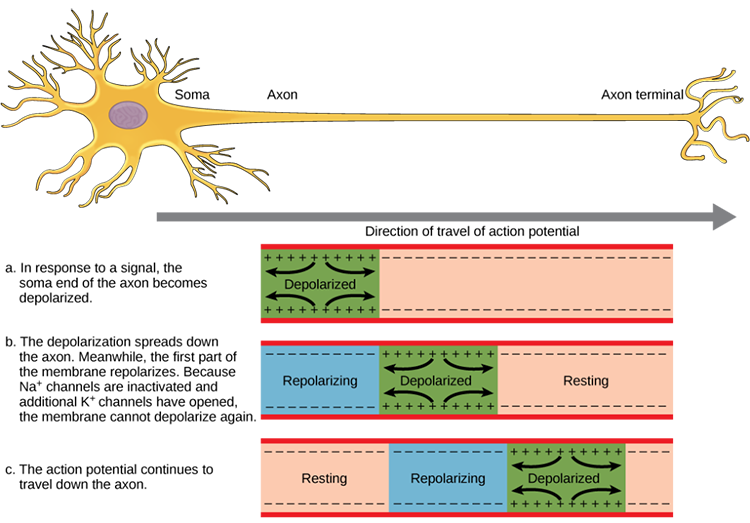
We can compare action potential conduction to the movement of water through a leaky garden hose.
Garden hose: water can take two paths, inside the hose or through holes in its wall, and the majority of the water will flow where movement is easiest. For a small-diameter hose with many large holes, most of the water will travel through the leaks. Conversely, for a large-diameter hose with only a few small holes, the bulk of the water will remain inside.
Axon: positive charge can take two paths, inside the axon or through pores in its membrane. Like water, a positive charge will take the path of least resistance. For a small-diameter axon with many open sodium ion channels, the majority of the current will exit the axonal membrane to the extracellular fluid. Small diameter, unmyelinated axons transmit action potentials without weakening since sodium ion channels constantly regenerate this signal. This method is slow because the signal travels step-by-step, small segment by small segment, and waits for sodium channels to admit enough positive ions to reach the excitation threshold. Check out the Blausen Continuous and Saltatory Propagation animation. Graphic © 2003 Josephine Wilson.
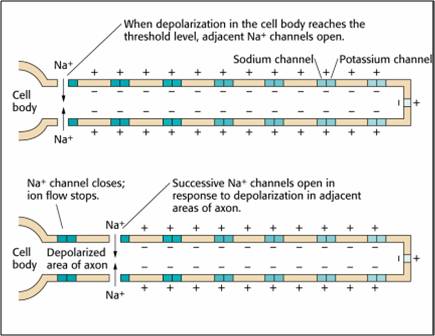
This method also consumes considerable energy since sodium-potassium transporters, powered by ATP, are located across the axon membrane to exchange three sodium for two potassium ions.
Conversely, for a large-diameter axon with few open ion channels, the bulk of the current will remain inside the axon's interior. Wider spacing between adjacent ion channels means that the action potential can depolarize a longer axon segment, which increases conduction velocity (Bear, Connors, & Paradiso, 2020).
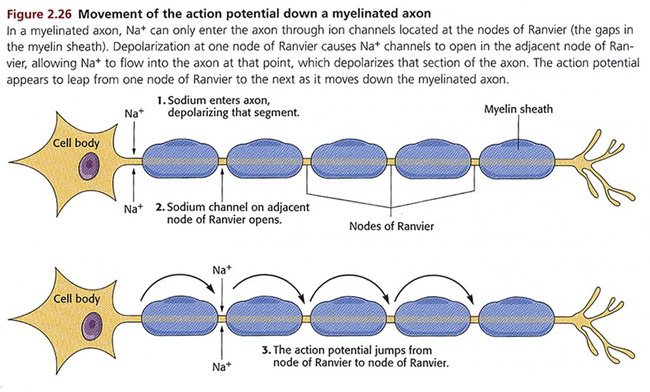
Medium-to-large diameter myelinated axons transmit action potentials using a method called saltatory conduction. Each segment of insulating myelin is almost 1-mm long. The gaps between segments, called nodes of Ranvier, are 1 to 2 thousandths of a millimeter. An action potential weakens under each myelinated segment (cable properties) and is then regenerated at each Ranvier node. The destruction of this insulation by demyelinating diseases like multiple sclerosis (MS) can be devastating because it disrupts neuron-to-neuron communication.
Saltatory conduction can be 200 times faster because the action potential jumps from node to node, in 1-mm steps,” instead of steps that are a thousand times smaller. This method is also more energy-efficient because sodium-potassium transporters are only needed at the nodes of Ranvier, where ion exchange is possible. These transporters account for about 40% of a neuron’s energy expenditure (Breedlove & Watson, 2020; Garrett, 2003). Graphic © 2003 Josephine Wilson.
Communication Between Neurons
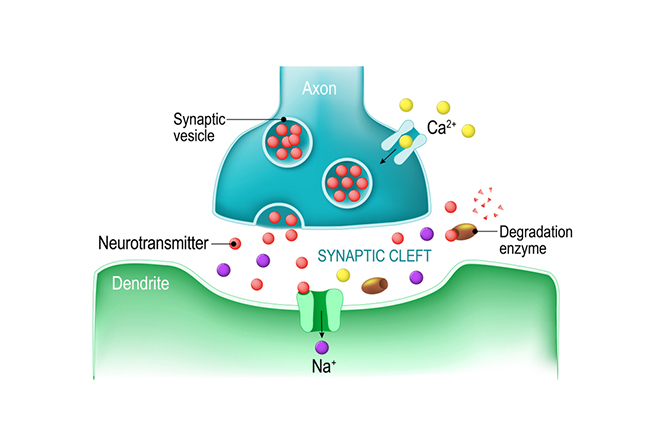
Neurons communicate through the release of neurochemicals and ions. Axon terminal buttons release neurochemicals across a 20-40-nm fluid-filled gap between presynaptic and postsynaptic structures called a synaptic cleft and into the extracellular fluid surrounding the neuron. Chemical synapses produce short-duration (millisecond) and long-duration (seconds to days) changes in the nervous system. Check out the YouTube videos, Neurotransmitter Synapse 3D animation and Neuronal Synapses.They are functionally asymmetrical because the presynaptic neuron sends a chemical message and the postsynaptic neuron receives it. They are structurally asymmetrical because the presynaptic element (axon) contains vesicles containing neurotransmitters, and the postsynaptic element (dendrite) doesn’t. The release of neurochemicals when an action potential arrives at the terminal button is called exocytosis. Graphic © Christoph Burgstedt/Shutterstock.com.
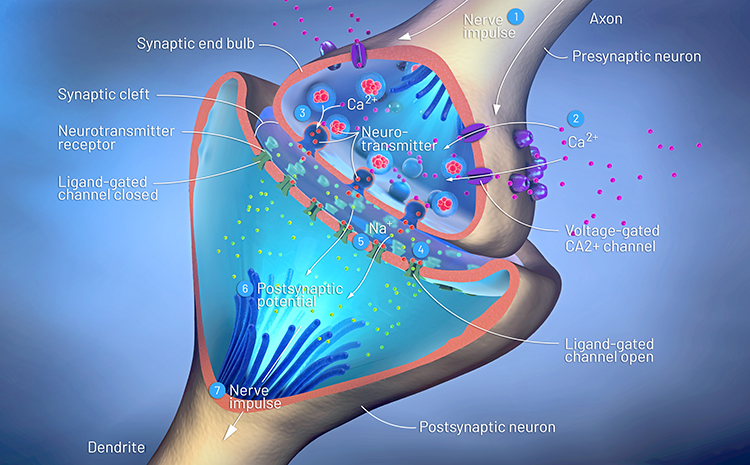
Old-school view: according to Dale’s law, a neuron can only release one neurotransmitter at a synapse.
New-school view: neurons can release a classical neurotransmitter and a peptide.
Old-school view: axon terminals only release neurotransmitters into the synaptic cleft.
New-school view: Neurotransmitter release also occurs outside of the synaptic cleft. Axonal varicosities (swellings in axon walls), dendrites, and the terminal button can release transmitters into the extracellular space. Graphic © 3Dme Creative Studio/Shutterstock.com.
Neuromodulation via Axoaxonic Synapses
Axons can influence the amount of neurotransmitters released when an action potential arrives at an axon terminal through axoaxonic synapses (junctions between two axons). Graphic © BruceBlaus.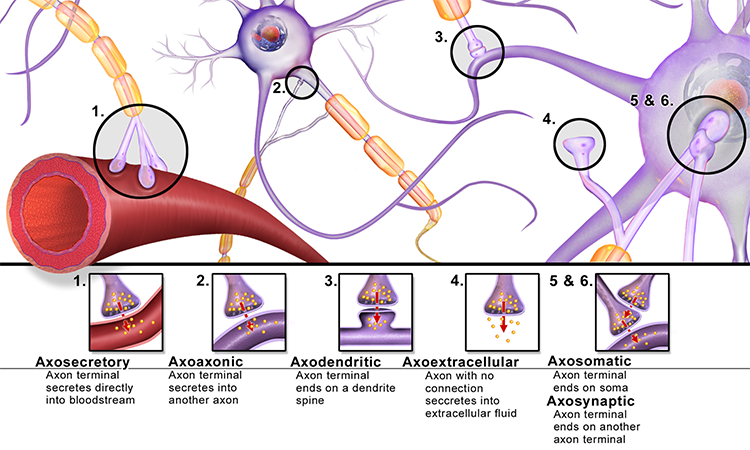
Axoaxonic synapses do not affect the generation of an action potential, only the amount of neurotransmitter distributed. In presynaptic facilitation, a neuron increases the presynaptic neuron's neurotransmitter release by delivering a neurotransmitter that increases calcium ion entry into its terminal button. In presynaptic inhibition, a neuron decreases neurotransmitter release by reducing calcium ion entry. These modulatory effects are confined to a single synapse (Breedlove & Watson, 2020).
Types of Neurotransmitters
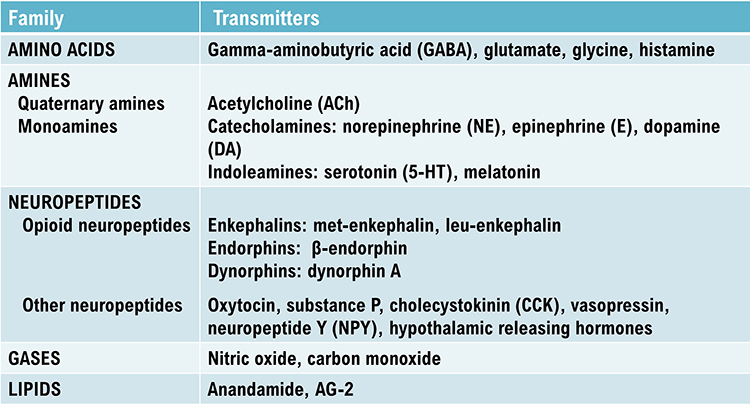
While the actual number of neurotransmitters is not known, more than 200 molecules have been identified. Each neurotransmitter may have multiple receptors. A neurotransmitter's effect, excitatory or inhibitory, depends on its interaction with specific receptors. The same neurotransmitter can produce opposite results at different receptor subtypes (Breedlove & Watson, 2020).The principal neurotransmitter families include amino acid neurotransmitters (GABA, glutamate), amine neurotransmitters (acetylcholine, dopamine serotonin), peptide neurotransmitters, also called neuropeptides (oxytocin, vasopressin), gas neurotransmitters (nitric oxide, carbon dioxide), and lipid neurotransmitters (anandamide and AG-2). The table below is adapted from Breedlove and Watson (2020).
Neurotransmitter Pathways
Researchers have identified pathways for acetylcholine, dopamine, norepinephrine, and serotonin. The reproduced diagrams are © Vasilisa Tsoy/Shutterstock.com.Cholinergic pathways
.jpg)
Cholinergic cell bodies and their projections originate in the basal forebrain and brainstem. Cholinergic pathways are involved in arousal, attention, memory, motivation, muscle contraction, and sleep. Watch the Blausen Chemical Synapse: Cholinergic Synapse animation.
Dopaminergic pathways
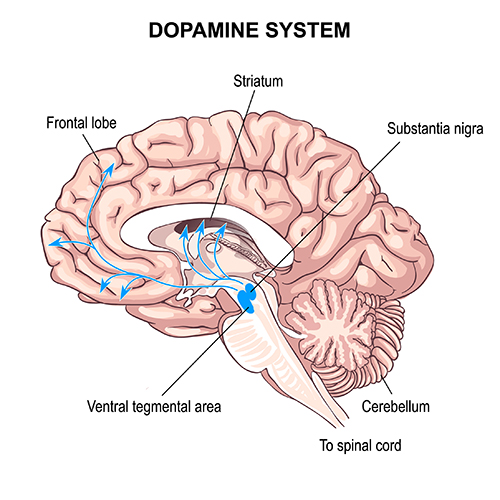
Two major dopaminergic pathways originate in the midbrain: the mesostriatal and mesolimbocortical pathways. Dopaminergic pathways are involved in addiction, motor control, and salience (reward- and threat-based motivation). Check out the Blausen Parkinsons Disease animation.
Noradrenergic pathways
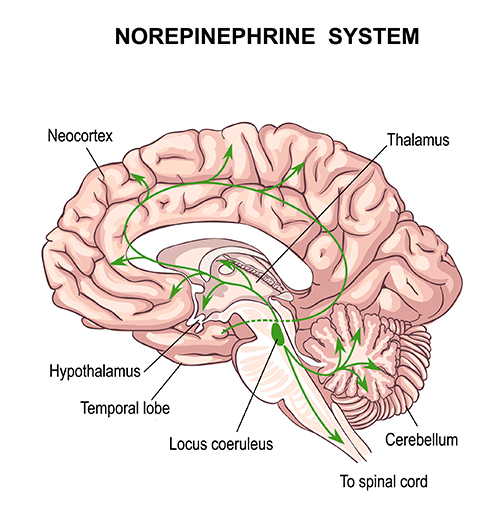
The noradrenergic pathways originate in the midbrain locus coeruleus and lateral tegmental area. Noradrenergic pathways are involved in arousal, attention, memory, vigilance, sleep, and mobilizing the brain and body for action, including the fight-or-flight response.
Serotonergic pathways
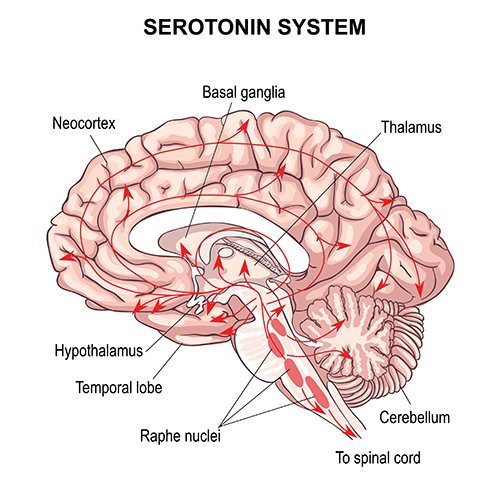
The serotonergic pathways originate in the brainstem and midbrain raphe nuclei. Serotonergic pathways are involved in appetite, mood, and sleep.
Termination of Neurotransmitter Action
Following exocytosis, neurotransmitter action is terminated by reuptake and enzymatic degradation. In reuptake, reuptake transporters located in the presynaptic terminal and astrocytes that enclose the synapse return neurotransmitter molecules to the presynaptic neuron. Astrocytes remove glutamate from the synapse. Graphic © Blamb/Shutterstock.com.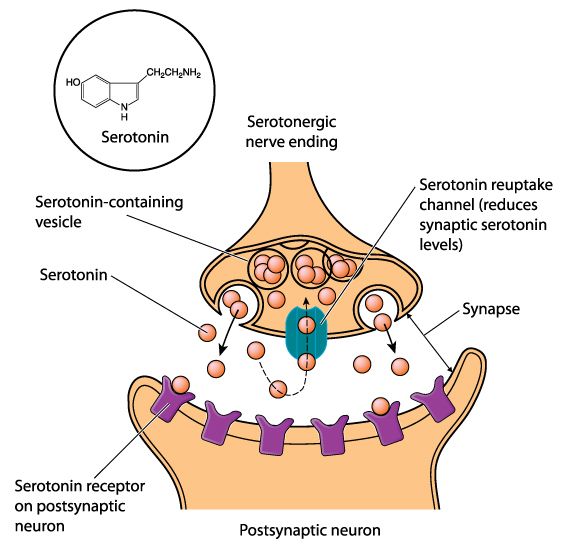
In enzymatic degradation, enzymes located in the synaptic cleft and the cytoplasm of the presynaptic neuron's terminal button split neurotransmitter molecules apart (e.g., acetylcholine). Graphic © Designua/Shutterstock.com.
Electrical Synapses
Electrical synapses communicate information across gap junctions between adjacent membranes using ions. Gap junctions are narrow spaces between two cells bridged by connexons (protein channels) that allow ions near-instantaneous travel. Graphic courtesy of Wikimedia Commons.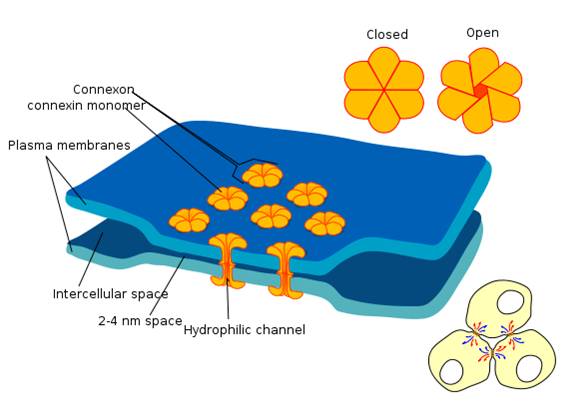
Electrical synapses are symmetrical. Ions flow across a 3-nm gap junction into the more negatively charged neuron as long as the gap junction remains open. This means that whether neurons are presynaptic or postsynaptic depends on their respective charges. When two neurons are electrically coupled, an action potential in one induces a postsynaptic potential (PSP) in the paired neuron.
Transmission across electrical synapses is instantaneous, compared with the 10-ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of neurons to synchronize their activity and simultaneously fire.
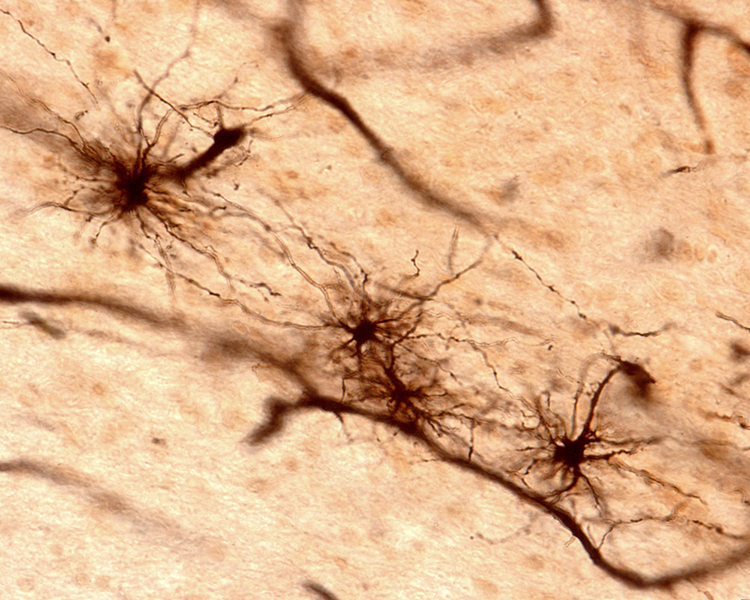
Astrocytes (shown below) communicate with each other and with neurons using gap junctions. Neurons that secrete hormones use electrical synapses to release their chemical messengers simultaneously. Neonatal brains may use gap junctions to activate many neurons at once. Image of long, fibrous astrocyte processes using Golgi's silver chromate technique © Jose Luis Calvo/Shutterstock.com.
Gap junctions may be a preliminary step toward developing chemical synapses between these neurons, eventually replacing their electrical synapses. Prenatally and postnatally, gap junctions enable nearby neurons to coordinate their development by sharing electrical and chemical communications (Bear, Connors, & Paradiso, 2020; Breedlove & Watson, 2020).
Old school view: synapses are either electrical or chemical.
New school view: synapses can be both electrical and chemical
Cortical Architecture
While no one has counted the neurons in the human nervous system, a recent estimate is that an adult brain contains about 86 billion neurons (Voytek, 2013). Each neuron connects with an average of 40,000 synapses. There are 10 times more glial cells than neurons, and they comprise 50% of the brain’s volume (Breedlove & Watson, 2020). The 2 trillion glial cells are considerably smaller than neurons, with somas between 6 to 10 μm in diameter (Hammond, 1996). Animation © nmlfd/iStockphoto.com.The cerebral cortex comprises neuronal cell bodies, glial cells, and blood vessels. Beneath the neocortex lies myelinated nerves (white matter), unmyelinated fibers, and glial cells.
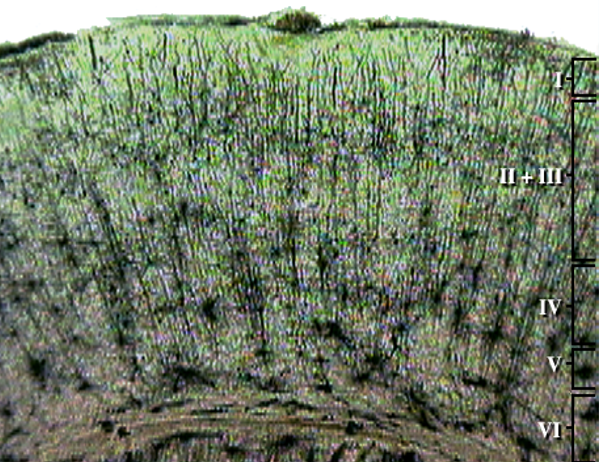
The cerebral cortex covers the cerebral hemispheres and consists of gray and white matter. Gray (or grey) matter, which looks grayish brown, comprises cell bodies. White matter gains its opaque white color from myelinated axons. The cerebral cortex of a macaque brain is shown below, courtesy of Wikipedia. Note that staining imparts a darker shade to gray matter.
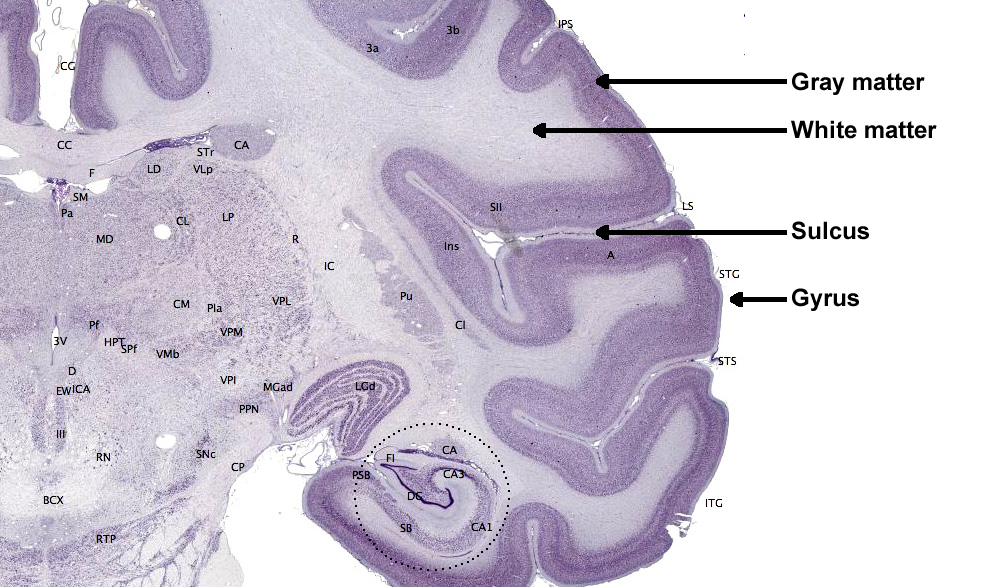
The convolutions of the cerebral cortex contain two-thirds of its surface area and maximize the volume of cortical tissue housed within the skull. Cerebral cortical convolutions include sulci, which are shallow grooves in the surface of the cerebral hemisphere (central sulcus), fissures, which are deep grooves (lateral fissure), and gyri, which are ridges of cortex demarcated by sulci or fissures (precentral gyrus) (Carlson & Birkett, 2021).
There are two main types of cortex: neocortex and allocortex.
The neocortex or isocortex consists of six layers 3 mm thick with a surface area of about 2360 cm2 with white matter underneath. Layers I-III receive corticocortical afferent fibers that connect the left and right hemispheres. Layer III is the main source of corticocortical efferent fibers. Layer IV is the primary destination of thalamocortical afferents and intra-hemispheric corticocortical afferents. Layer V is the primary origin of efferent fibers that target subcortical structures that have motor functions. Layer VI projects corticothalamic efferent fibers to the thalamus, which together with the thalamocortical afferents, creates a dynamic and reciprocal relationship between these two structures (Creutzfeldt, 1995). Diagram courtesy of Wikimedia Commons.
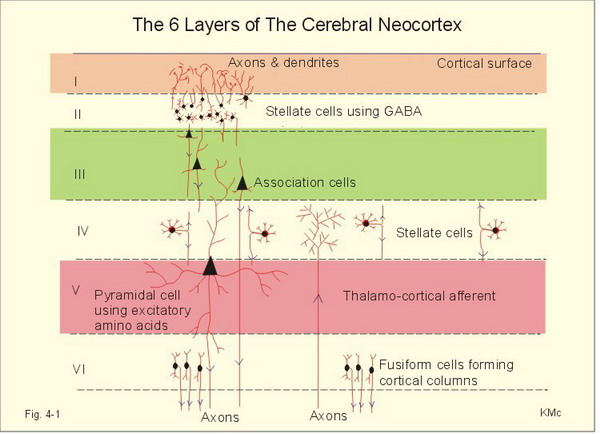
Allocortex, which means other cortex, usually has between three or four layers, compared with the neocortex's six layers. The allocortex has less volume than the neocortex and comprises the olfactory system and hippocampus.
A transitional region between the neocortex and allocortex is called the paralimbic cortex.
For a basic overview of the cortex, watch the Khan Academy video Cerebral Cortex.
Neurons in the Cortex
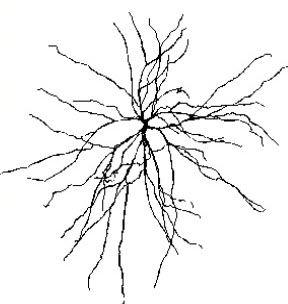
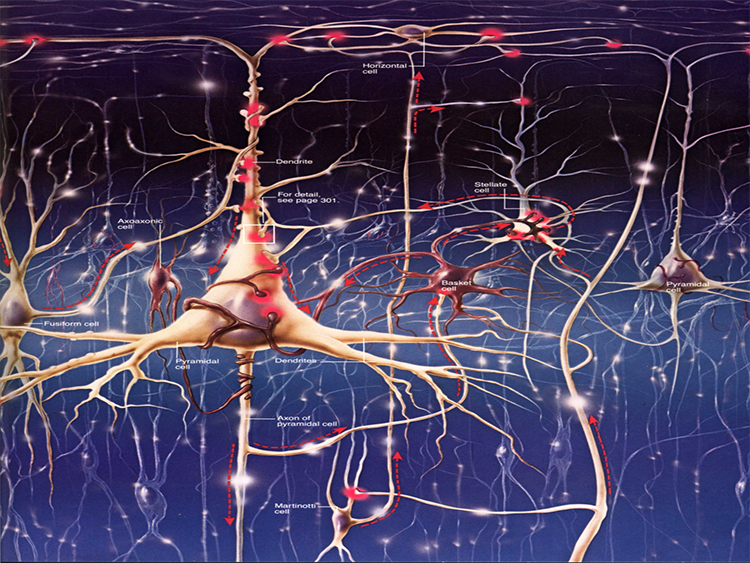
We can classify cerebral cortical neurons as whether their dendrites display spines or not. Spiny neurons, which have either pyramidal or stellate (star-like)-shaped cell bodies, are usually excitatory. While all pyramidal cells are spiny neurons, stellate cells can be spiny or aspinous (Bear, Connors, & Paradiso, 2020).The graphic below depicts dendritic spines.
There are many types of aspinous (smooth) neurons, which are believed to be inhibitory.
What is the EEG?
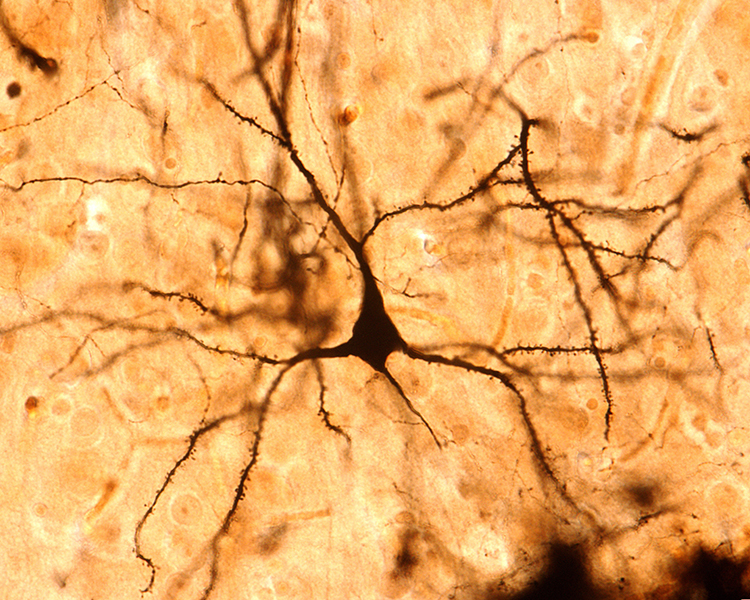
The scalp EEG is the voltage difference between two recording sites recorded over time. The EEG is primarily generated by large pyramidal neurons located in layers 3 and 5 of the 2-4.5-mm-thick cortical gray matter. Image of a pyramidal neuron revealed using Golgi silver chrome © Jose Luis Calvo/Shutterstock.com. Note that the apical dendrite arising from the cell body and basilar dendrites feature an extensive network of spines.
Local activity is a composite of local and network influences. Network communication systems and local cortical functions show different characteristics across the cortex and produce unique and specific EEG patterns in other regions.
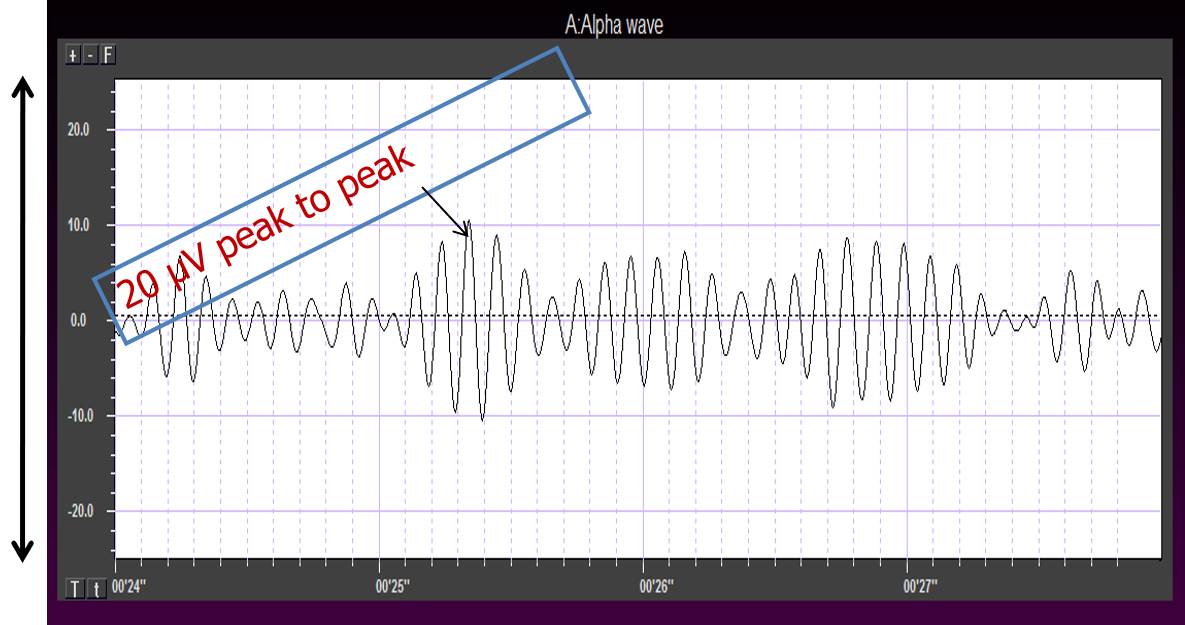
The movie below is a display of the raw EEG with voltage shown as μV peak to peak © John S. Anderson.
What Can the EEG Tell Us?
With the EEG, we can follow the progression from stimulus to behavior response. This allows us to determine the correct function at each step and identify causal factors in dysfunctional outcomes or responses.Source of the Scalp EEG
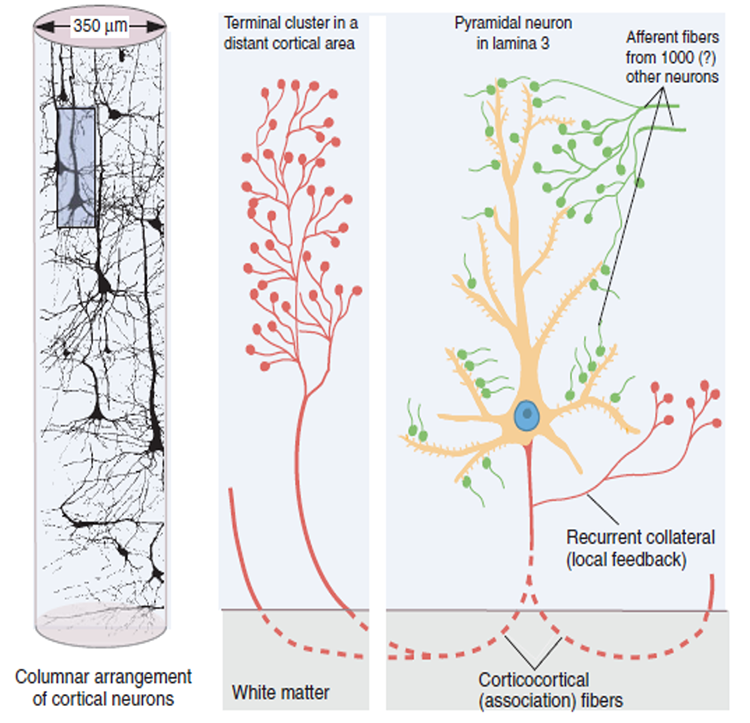
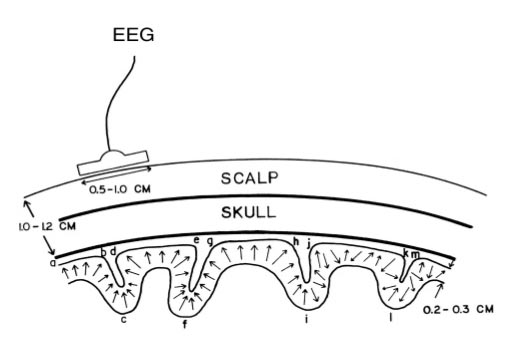
The scalp EEG results from the summation of large areas of gray matter activity. Areas are polarized synchronously due to the input of oscillatory or transient evoked activity. These areas comprise thousands of cortical columns containing large pyramidal cells aligned perpendicularly to the cortical surface.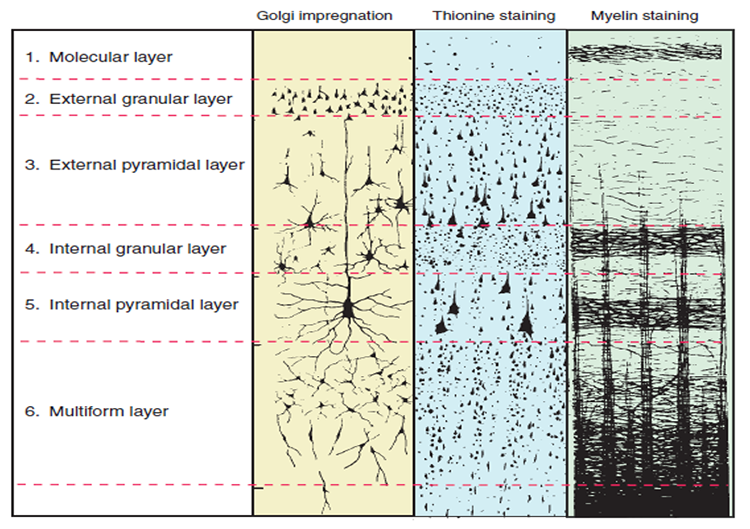
Pyramidal neurons are found in all cortical layers, except layer 1, and represent the primary type of output neuron in the cerebral cortex.
The scalp EEG results from the summation of EPSPs and IPSPs in thousands of cortical columns containing large pyramidal cells perpendicular to the cortical surface. The columns are synchronously polarized (made more negative) and depolarized (made less negative) due to the input of oscillatory or transient evoked activity.
Local Field Potentials
The local field potential (LFP) is the aggregate effect of the firing of the interconnected pyramidal neurons within the cortical columns, plus additional mechanisms like glial cell modulation of the cortical electrical gradient.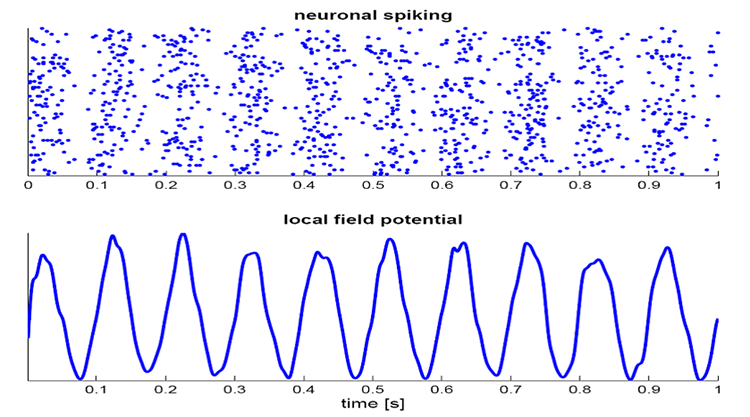
Caption from Wikipedia's article on Neural Oscillation. Simulation of neural oscillations at 10 Hz. The upper panel shows spiking of individual neurons (with each dot representing an individual action potential within the population of neurons). On the lower panel, the local field potential reflects their summed activity. This figure illustrates how synchronized patterns of action potentials may result in macroscopic oscillations that can be measured outside the scalp.
Do not confuse the "spiking" of individual neurons with epileptogenic spikes in the scalp EEG.
Scalp Electrical Potentials
Scalp electrical potentials represent the sum of all available electrical fields. Fields of opposite polarity (+/-) cancel each other out so that scalp potentials are greater when large aggregates of neurons polarize and depolarize synchronously. The scalp EEG represents a weighted sum of all active currents with the brain that generate open fields, including non-cortical sources.Action potentials reflect neuronal output. They are seen in extracellular recordings as fast (~300 Hz) activity that exceeds 90 mV lasting less than 2 ms. Action potentials play a minor role in scalp surface EEG. They fall below 60 V outside of a 50-μm (0.050-mm) radius. Scalp electrodes are several centimeters from cortical neurons and are generally aligned away from the scalp. Therefore, action potentials are unlikely to contribute significant voltages to the scalp EEG.
Local Field Potentials Regulate Neuron Excitability and Firing
Neurons are most likely to fire during the depolarizing phase of the local field potential. Neurons are more excitable when they are "in phase" with respect to the local field potential (LFP) and are inhibited when they are out of phase with the LFP. Thus, at any instant of time, the amplitude and frequency of the EEG are regulated by the LFP, which in turn, is influenced by oscillatory mechanisms such as slow cortical potentials.The movie is a 19-channel display of SCPs © John S. Anderson. Negative SCPs drift down, and positive SCPs drift up. SCPs represent a global shift in DC voltage across the cortex and represent a generally higher (negative SCPs) or lower (positive SCPs) state of cortical excitability regulating neural networks.
The EEG is a moment-to-moment measure of the excitability of action potential firing, like gates opening and closing on the half cycle.
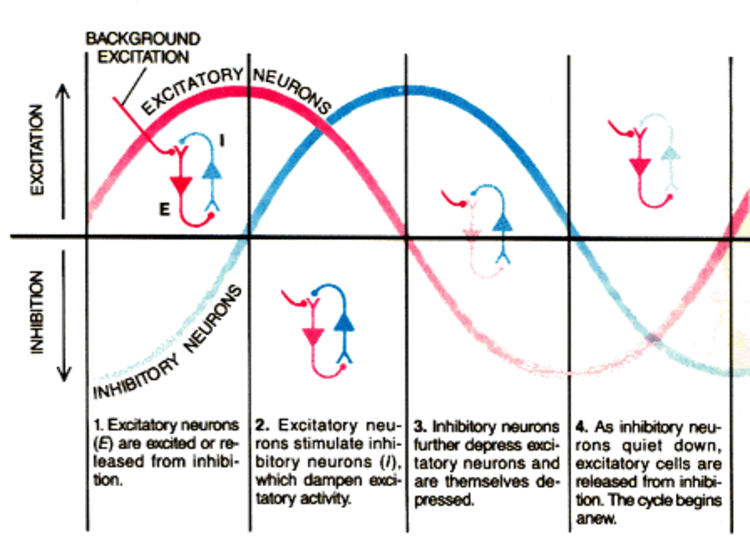
The synchronous activity of large pyramidal neurons networked in cortical columns creates the EEG.
The Composition of the EEG
The EEG is composed of electrical potentials, varying in two dimensions, frequency and amplitude.
Sources of IPSP and EPSP Inputs
Many sources contribute input that results in IPSP and EPSP activity within cortical neurons. These sources primarily contribute influences such as oscillatory generator input or ascending event-related evoked input.EEG Sources
Generators like the thalamus produce oscillatory activity among many interconnected neurons, including EEG patterns like the alpha rhythm.
Movie © John S. Anderson. The recording begins with eyes open. The eyes-closed condition starts at 14’01” and clearly shows increased 8-12 Hz voltage (posterior dominant rhythm or PDR) in occipital and parietal locations in the line tracing and topographic maps to the right of the tracing.
The eyes open again at 14’31”, and alpha attenuates (alpha blocking). This shows the posterior dominant rhythm (generally known as "alpha") appearing in the eyes-closed condition when visual sensory input is stopped. The attenuation or blocking of this rhythm as sensory input returns in the eyes-open condition.
The thalamus contributes to slow cortical potentials, 1-4 Hz delta, 8-12 Hz alpha, and 20-38 Hz beta (including 40-Hz activity). The diagram shows the connections between the pulvinar (bottom right) and reticular nuclei (bottom left) of the thalamus and the cortex © Elsevier Inc. - Netterimages.com.
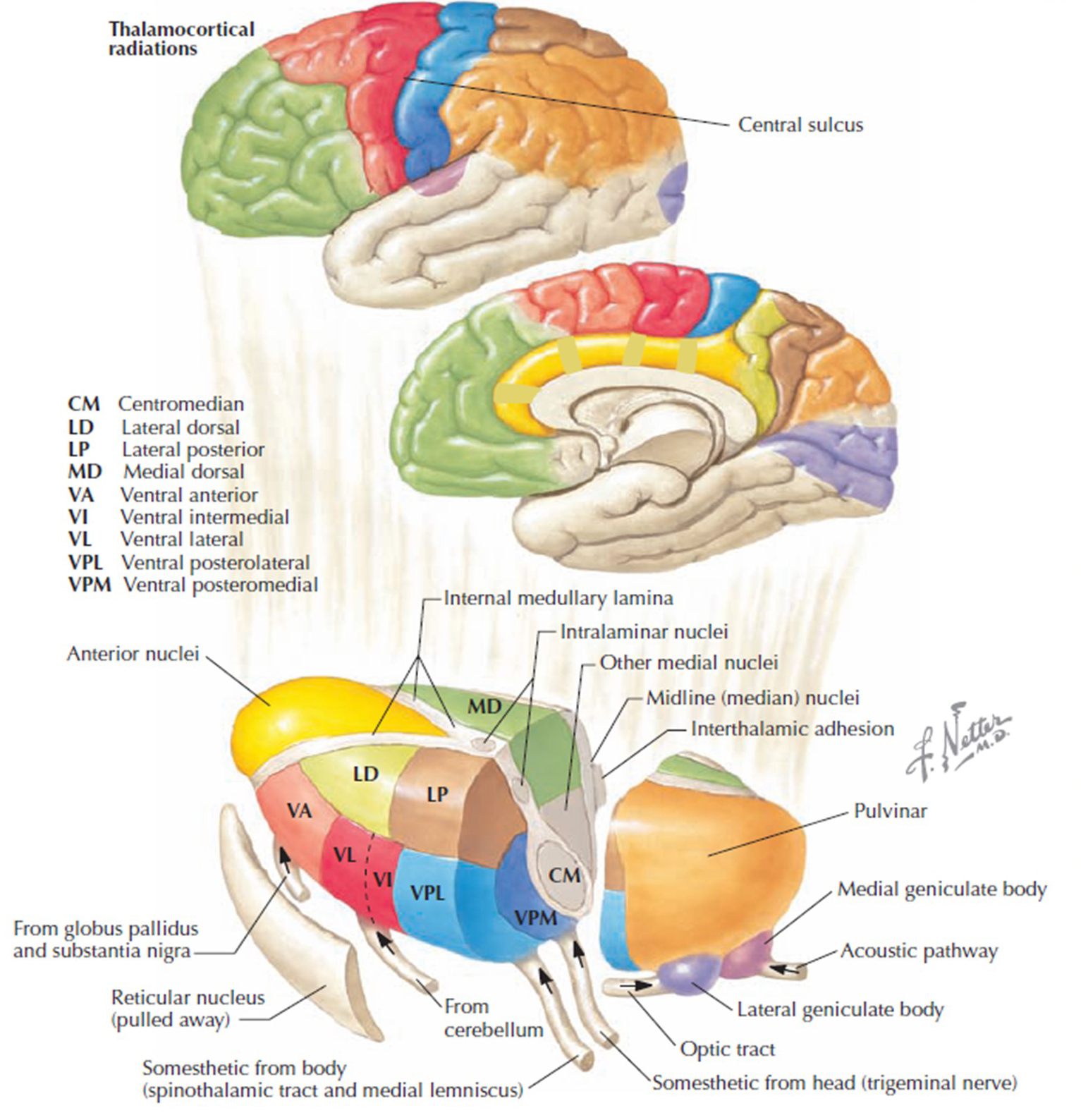
The diagram below, which shows bidirectional connections between the thalamus and cortex, was modified from the original on www.lib.mcg.edu.
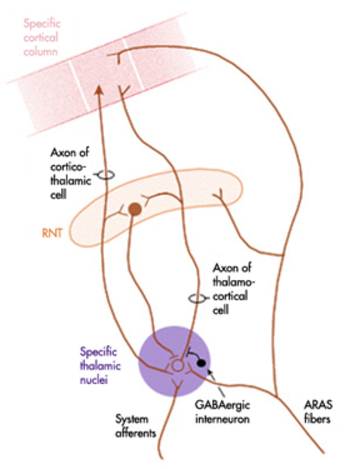
Caption by W. D. Jackson, PhD, and S. D. Stoney, PhD (2006): Thalamocortical cells are subject to excitatory drive from their system afferents, from monosynaptic corticothalamic fibers, and from the brainstem reticular formation (ascending reticular activating system, ARAS). They receive inhibitory drive from local interneurons and neurons in the reticular nucleus of the thalamus (RNT). Note that the RNT neurons are excited by activity in thalamocortical cells and corticothalamic cells. The connections are precisely organized. For example, each column in a primary cortical area sends corticothalamic fibers back to the same part of its specific thalamic nucleus that sends its thalamocortical fibers to that cortical column. The corticothalamic fibers also synapse on the RNT cells receiving input from that part of the thalamic nucleus. Each cortical receiving area is said to be "reciprocally connected" with its specific thalamic nucleus. Like the thalamocortical cells, RNT cells and cortical neurons also receive excitatory drive from the ARAS.
The EEG is generated by thalamocortical (alpha) and cortical-cortical (beta) sources.
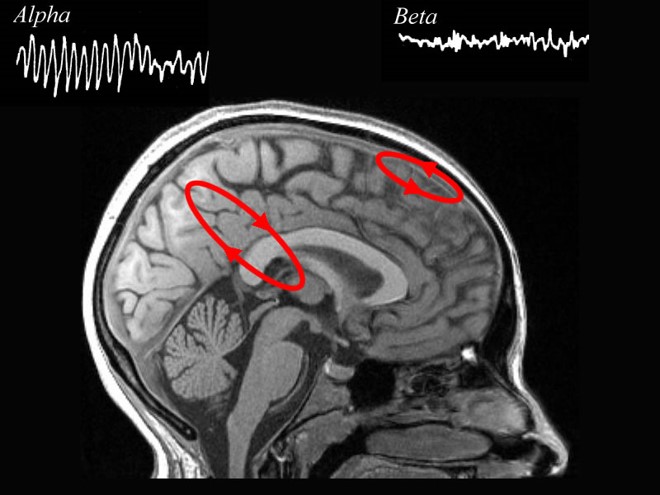
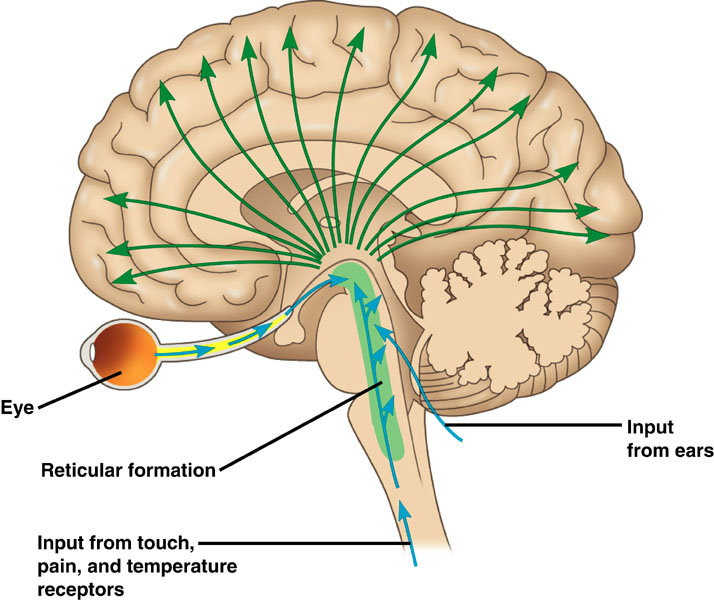
Neurons in the ascending reticular activating system produce event-related potentials in response to diverse stimuli like a flashing light or sound. Event-related potentials (ERPs) are the brain's response to externally applied stimuli, events, or cognitive/motor tasks. They are time-locked measures of brain electrical activity.
Dipole Generators
Large cortical pyramidal neurons organized in macrocolumns are oriented with an apical dendrite projecting toward the scalp and an axon descending in the opposite direction. An "Equivalent Dipole Generator" usually represents the sum of all multipolar current sources. Summed generators are modeled as dipoles to aid the conceptual understanding of the electrical fields involved.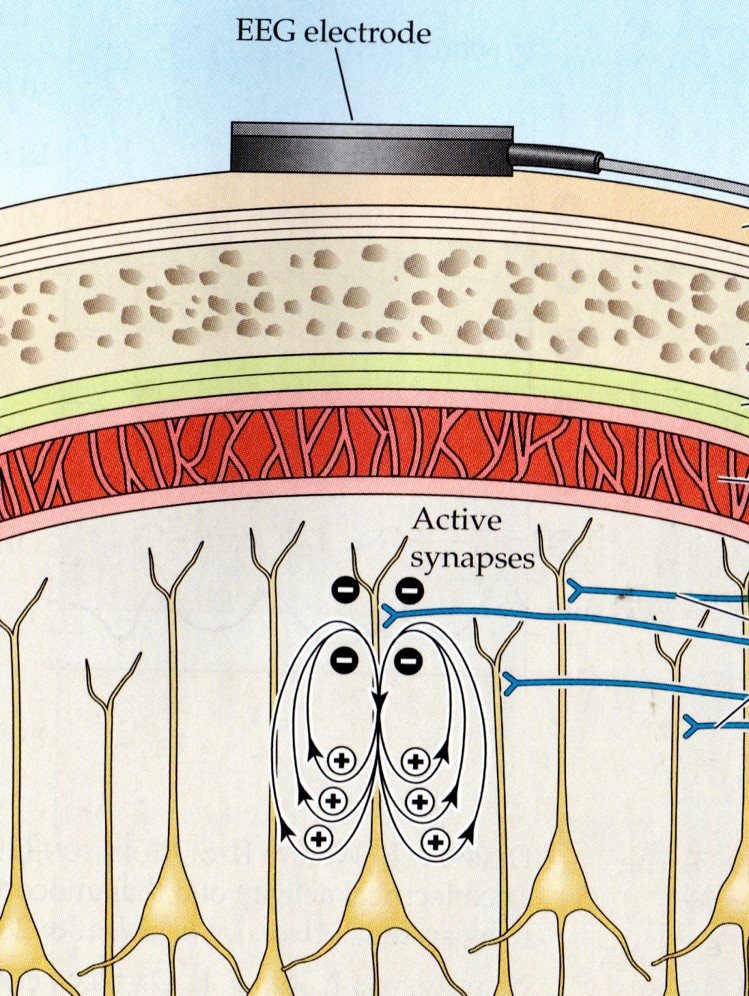
EEG Signals (Brainwaves)
The EEG represents changes in a brain area's electrical activity (potential) compared to a "neutral" site or another brain area. The EEG is displayed as oscillations or voltage fluctuations, which show a "wave" pattern when plotted on a graph.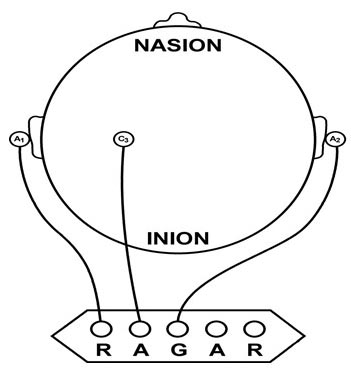
"These oscillations are generated spontaneously in several areas of the cerebral cortex as neuronal networks transiently form assemblies of synchronously firing cells." Klaus Linkenkaer-Hansen.
Sink, Source, and Dipole
We can describe pyramidal cells in terms of their sink, source, and dipole. A sink (-ve), which may be located at the bottom, middle, or top of the apical dendrite, is where positive ions enter the dendrite. Cation (positive ion) entry gives the extracellular space a negative charge. The source (+ve) is where the current exits the cell. Finally, the dipole is the field created between the sink and source (Thompson & Thompson, 2016).The graphic below from Euroform Healthcare: Conduction Studies depicts current entering the apical dendrite (sink) of a pyramidal neuron where an afferent neuron has generated an EPSP. The current leaves the neuron (source) from the dendrite or cell body.
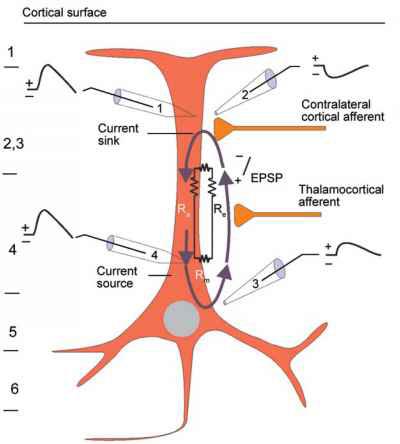
The postsynaptic potentials (EPSPs and IPSPs) propagated by the apical dendrites in layers 2 and 3 create an extracellular dipole layer parallel to the cortical surface. The dipole layer's electrical polarity is the opposite of the deeper cortical layers 4 and 5 (Fisch, 1999).
A cortical dipole is created when pyramidal neurons depolarize simultaneously. This phenomenon is called local synchrony. Fewer than 5% of pyramidal neurons can generate more than 90% of the power in the EEG signal because most pyramidal neurons usually fire asynchronously so that their potentials counteract each other. A small fraction of these neurons firing in step can produce visible changes in EEG feedback. This creates the potential for operant conditioning to help clients learn to modify EEG activity through neurofeedback.
Cortical dipoles have three properties: site (depends on source), size (oscillation frequency and voltage), and relative position with respect to sulci and gyri (Collura, 2014).
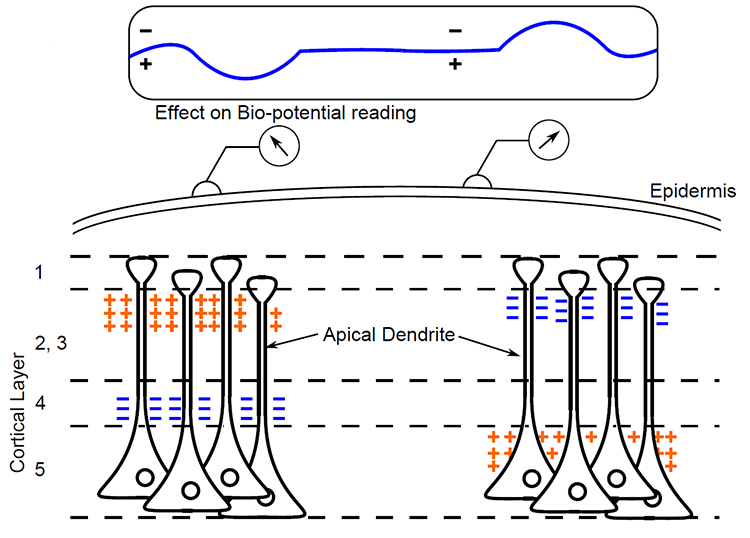
The EEG is Mainly Sensitive to Radially Oriented Dipoles
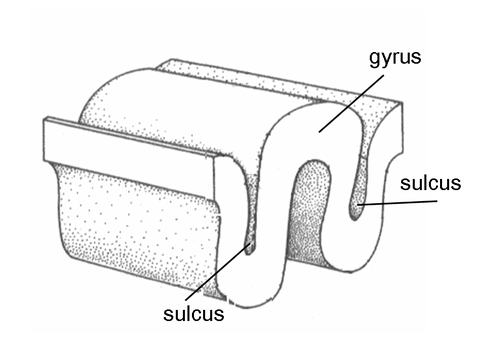
Evolution has convoluted the human brain to increase its computing power without enlarging the skull. This enfolding has created two easily visible anatomical features: gyri and sulci.
Recall that a gyrus is a ridge of the convoluted cerebral cortex, while a sulcus is a valley. The graphic below is courtesy of Wikipedia.com from the article Sulcus (neuroanatomy).
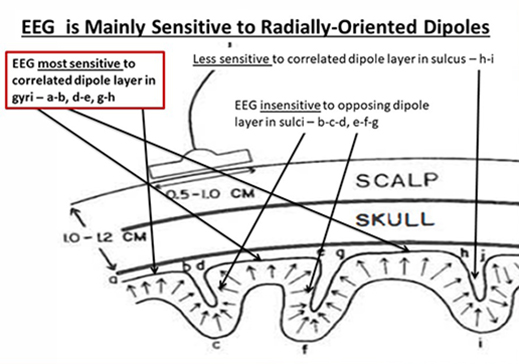
The EEG is most sensitive to a correlated dipole layer in gyri (a-b, d-e, and g-h). The EEG is less sensitive to a correlated dipole layer in sulci, valleys within the cortex (h-i). Finally, the EEG is insensitive to an opposing dipole layer in sulci (b-c-d, e-f-g). Graphic © Nunez (1995).
The EEG is composed of electrical potentials that vary along the dimensions of amplitude and frequency.
EEG Amplitude
The "amount" or amplitude and the "pattern" or morphology of any EEG frequency band reflect the number of neurons discharging simultaneously at that frequency. Lower neuron firing rates correspond to lower signal amplitude.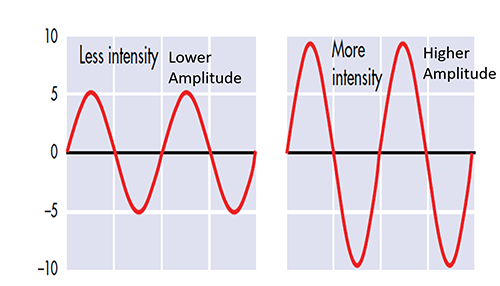
Amplitude measures the amount of energy in the signal and is usually expressed in microvolts.
Greater synchrony in firing among neurons results in higher amplitude, as shown with alpha in the graphic below.
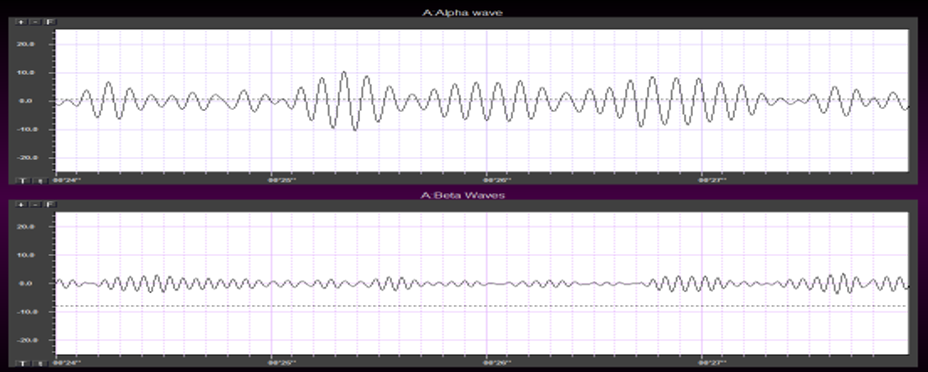
Greater firing synchrony produces larger EEG potentials that can be measured from the scalp surface.
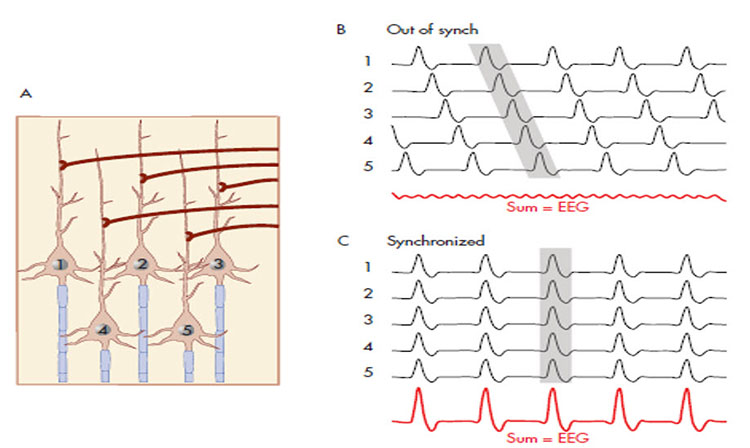
The EEG plots voltage changes over time, which can be displayed on a graph. The sampling rate is the number of measurements per second (Hz). Precision is the number of voltage gradations or steps.
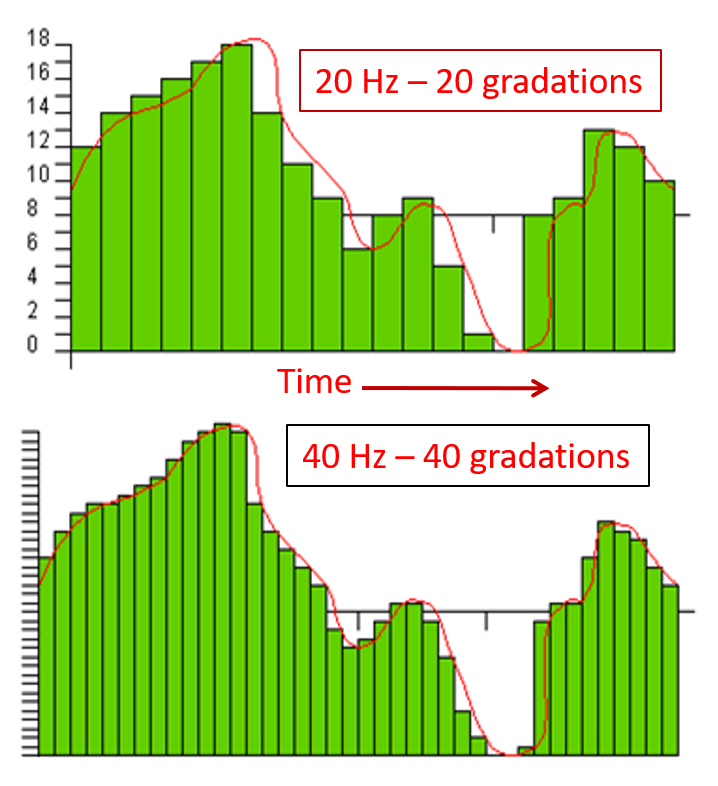
The analog-to-digital (A/D) converters that transform voltages into numerical values vary in precision: more bits correspond to greater accuracy. The graphic below shows precision differences using 12-14-bit (grey) and 20-24-bit A/D converters.
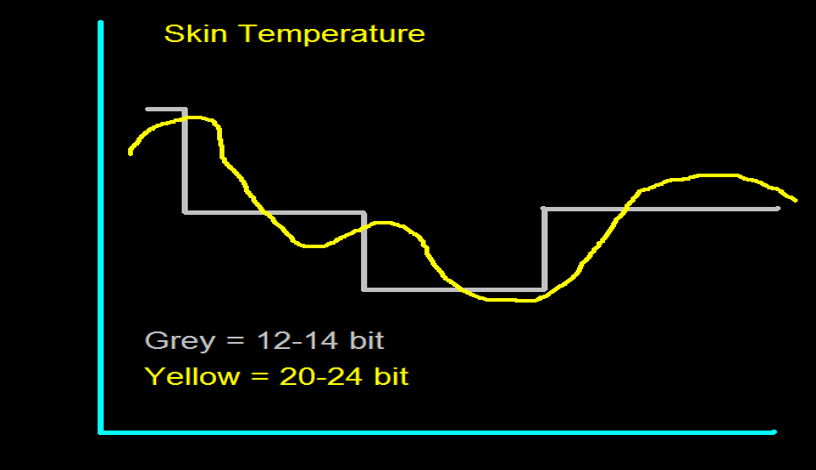
EEG Frequencies
The raw EEG contains all EEG frequencies, just as white light contains all light frequencies. Digital filters separate the EEG frequencies just as a prism separates individual colors. Graphic © kmls/ Shutterstock.com.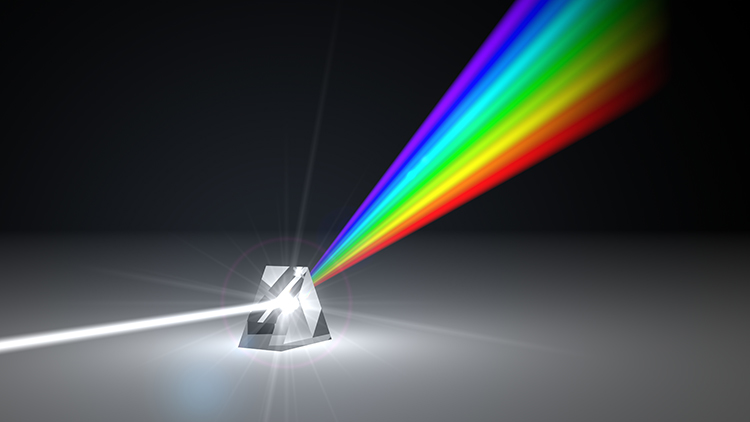
EEG frequency is measured in cycles per second or Hz. Count the number of peaks or count the number of zero (0.0) crossings divided by 2.
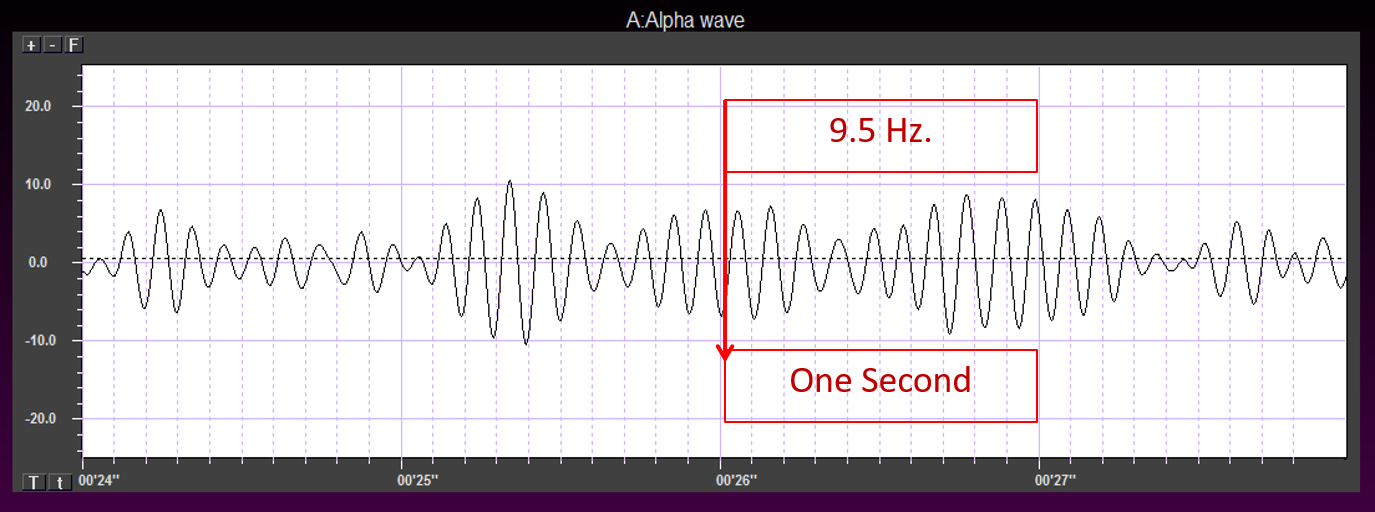
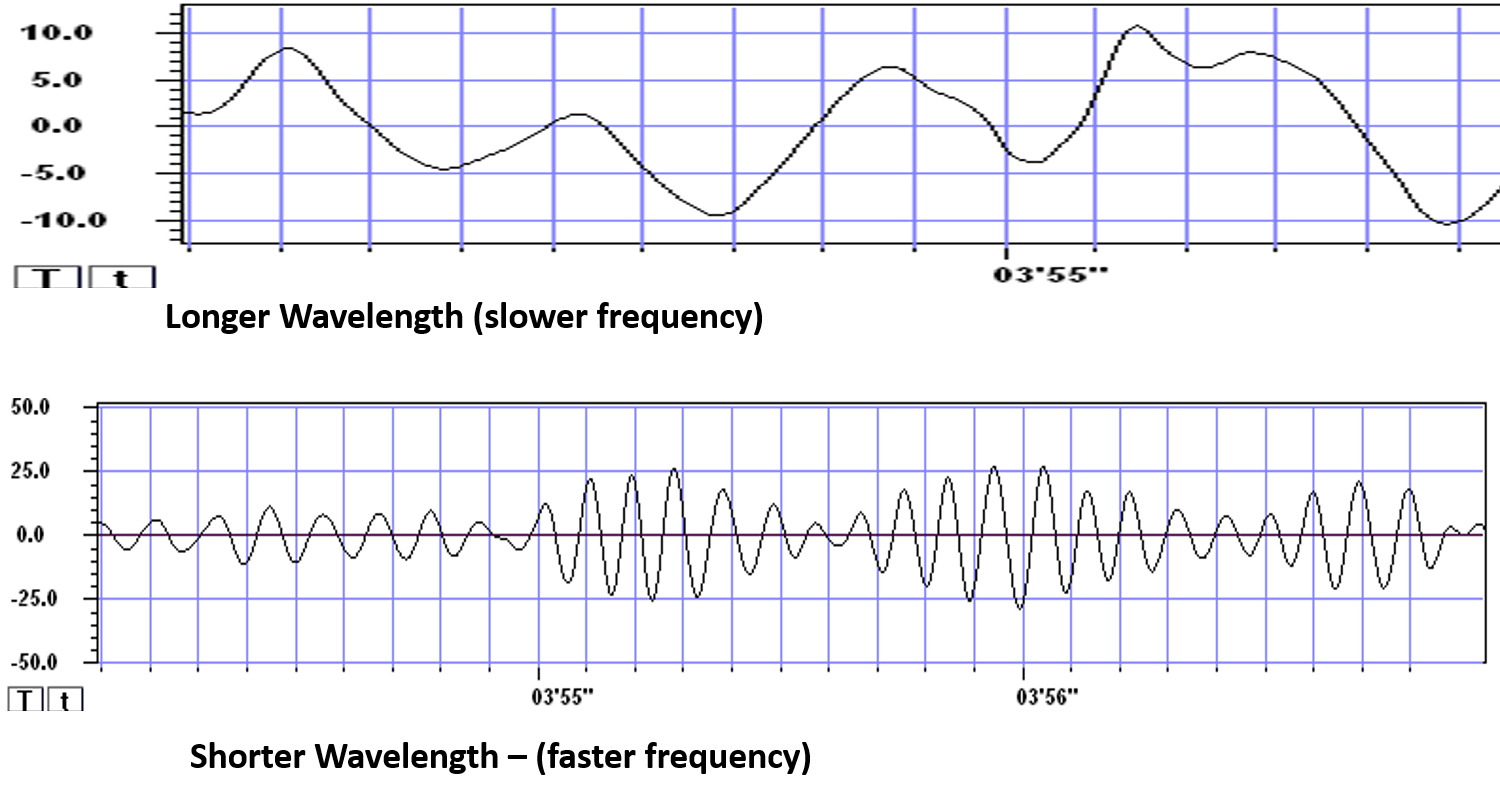
The slower the waves, the lower the EEG frequency.
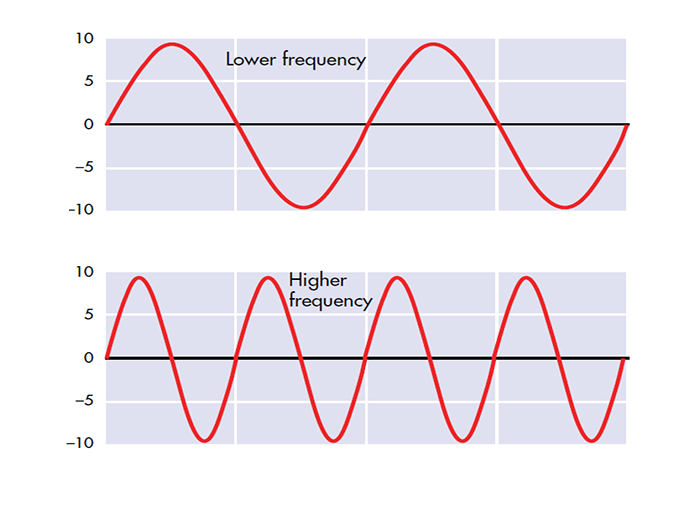
The longer the wavelength, the slower the frequency.
The movie is a 19-channel display of EEG activity from 1-64 Hz activity broken into its component delta, theta, alpha, and beta frequency bands by digital filters © John S. Anderson.
The movie is a 19-channel display of alpha activity © John S. Anderson. Brighter colors represent higher alpha amplitudes. Frequency histograms are displayed for each channel. Notice the runs of high-amplitude alpha waves.
EEG Oscillations
The generation of oscillatory activity, sometimes called spindle behavior, is likely due to the interaction between thalamocortical relay neurons (TCR), reticular nucleus neurons (RE), and interneurons. These interactions are mediated by diverse neurotransmitters, including acetylcholine and GABA.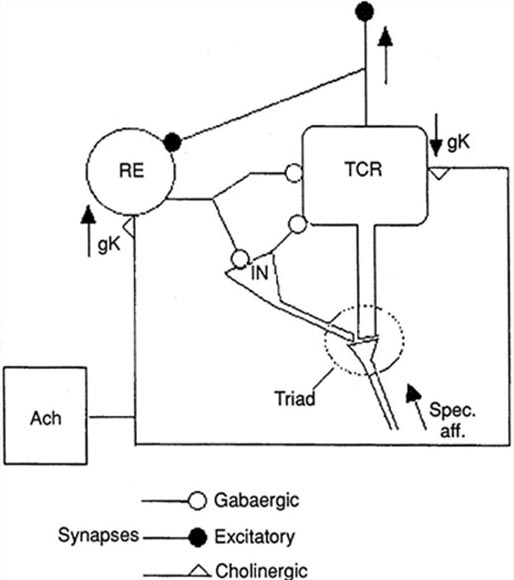
Circuits Contributing to the EEG
Feedforward, thalamocortical, and intra-cortical networks help generate the EEG. Graphic of circuits contributing to the EEG by Hindriks and van Putten © 2013 NeuroImage.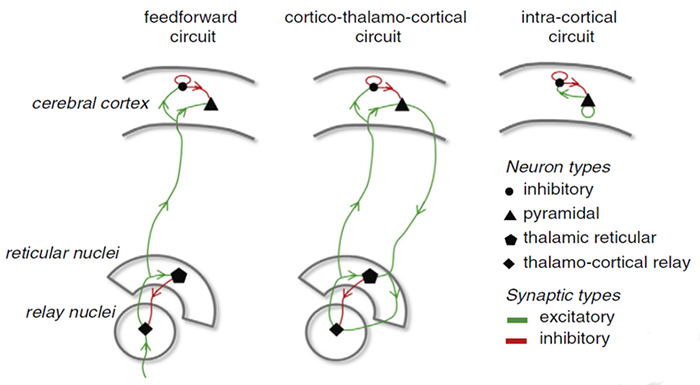
Spindling or Bursting Activity
Spindling is a synaptically-generated oscillation in a circuit that necessarily includes reticular nucleus neurons (RE).The movie below is a display of EEG spindling activity © John S. Anderson.
The various spindle frequencies, which have often been interpreted as reflecting different types of oscillations, merely depend on various durations of the hyperpolarizations (negative shifts) in thalamic-cortical relay neurons. Long duration hyperpolarizations, as during ... deeply EEG-synchronized states, are associated with 7 Hz or even lower-frequency spindles, while relatively short hyperpolarizations result in ... higher frequencies (14 Hz) (Steriade, 2005).
The Purpose of Oscillatory Activity
A single neuron can influence multiple postsynaptic targets located between 0.5 and 5 mm away with conduction periods of between 1 and 10 ms. This time difference becomes progressively more pronounced when more complex events involve progressively larger assemblies of neurons. It may take hundreds of thousands of neurons, stimulating multiple postsynaptic neurons, for the desired outcome to occur. When these many neurons are involved, it becomes increasingly clear that there is a need for organization and structure to manage this diverse activity.Timing is everything since action potentials arrive from a large number of sources. The nervous system must correctly register arrival times to recognize a face, recall a name, or remember personal history and context.
Hierarchical Processing
Complex events require that the systems involved operate within a spatial and temporal hierarchy. Each oscillatory cycle is a window of time within which processing can occur. Each cycle has a beginning, and an end within which encoded or transferred messages must complete their tasks. Groups of neurons, close or distant, interact most effectively when firing windows are synchronous. The brain does not operate continuously but in discontinuous packets. Graphic © Science by Knight (2007).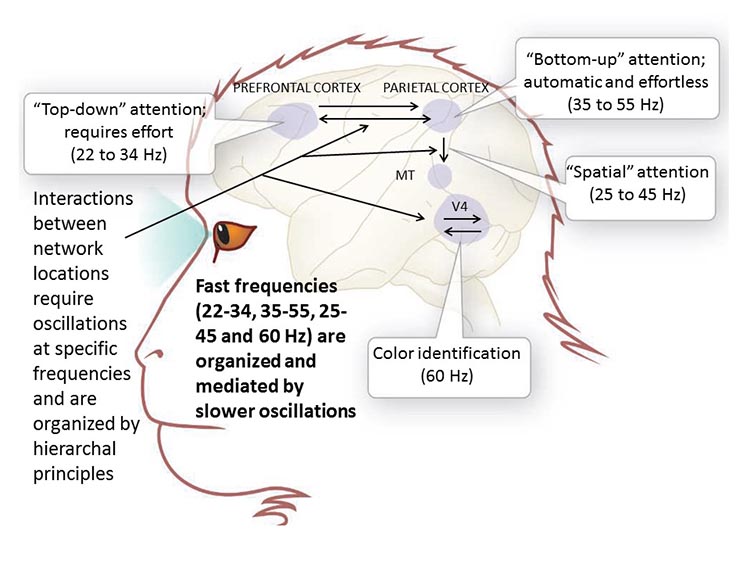
Multiple Oscillators
"Oscillatory classes in the cerebral cortex show a linear progression of the frequency classes on the log scale. In each class, the frequency ranges ('bandwidth') overlap with those of the neighboring classes, so that frequency coverage is more than four orders of magnitude" (Buzaki, 2006).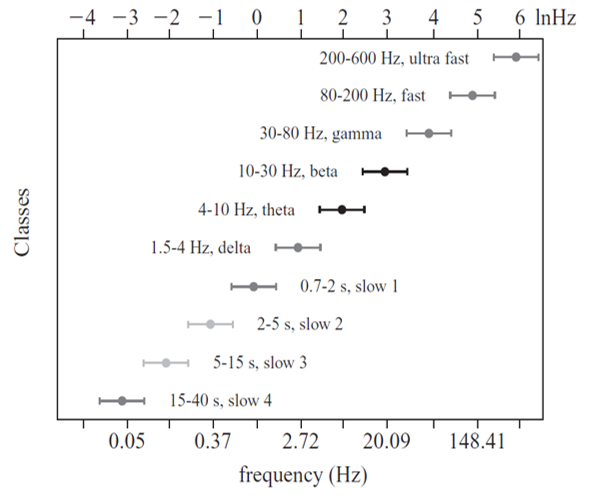
Frequency Determines Complexity
The wavelength or frequency of the EEG band determines how long the processing window will remain open and, therefore, the size of the neuronal pool involved. Because of the distances involved, longer wavelengths (slower frequencies) allow larger groups of more distant neurons to be assembled and coordinated. Different frequencies organize different types of connections and different levels of computational complexity.The graphic below © Trends in Cognitive Sciences by Ward (2003) illustrates the processing of memories of letters. One letter is refreshed during each gamma cycle, and memories are scanned at the gamma rate (frequencies above 30 or 35 Hz).
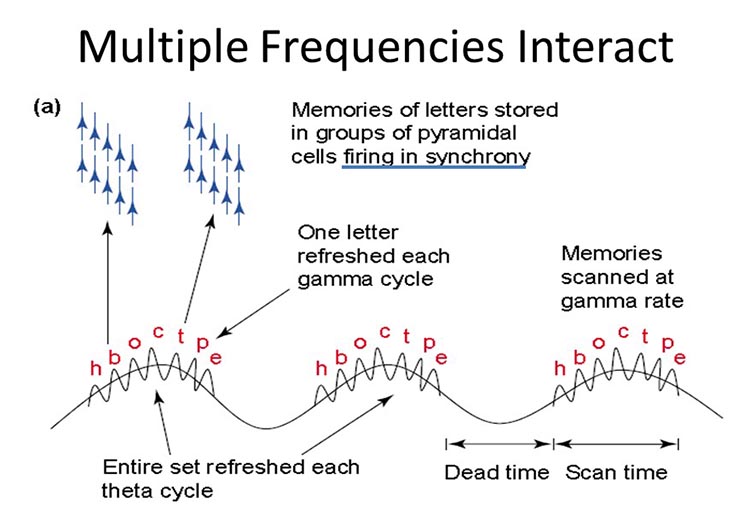
Local Versus Global Decision-Making
Short time windows of fast oscillators facilitate local integration, primarily because of the limitations of axon conduction delays. Fast oscillations favor local decisions. Slow oscillators can involve many neurons in large and/or distant brain areas. Slow oscillations favor complex, global decisions.Complexity Versus Frequency
Complex tasks involving sensory integration and decision-making were associated with 4-7 Hz synchronization. Intermediate tasks such as identifying spoken and written words and pictures increased 13-18 Hz beta activity. Simpler, more localized tasks, such as the visual processing of grid displays, were associated with faster-frequency activity (24-32 Hz) (Sarnthein et al., 1998; Von Stein et al., 1999).Traveling Waves Help Coordinate Widespread Brain Networks
Zhang et al. (2018) proposed that traveling waves between 2 to 15 Hz, moving at 0.25-0.75 meters per second across the cortex, mediate large-scale coordination of brain networks and support connectivity.Summary of EEG Oscillations
When the CNS processes incoming content, separate areas detect features of salient content, including visual, auditory, tactile, kinesthetic, and olfactory information. The CNS shares, integrates, compares current with previous content, analyzes, and makes decisions regarding memory and responses. Interacting networks linked by electrical and chemical signals perform this work. We record the electrical potentials generated by this complex and dynamic network activity as the EEG.The movie below of bursting alpha shows the sequential synchronization/desynchronization of groups of neurons. Higher voltage bursts are followed by voltage decreasing toward zero. These voltage fluctuations reflect rhythmic changes in the local field potential. This video © John S. Anderson.
DEFINITION OF ERPs AND SCPs
Sensory evoked potentials are a subset of event-related potentials (ERPs)
Event-related potentials (ERPs) represent the brain's responses to external stimuli, events, or cognitive/motor tasks. ERPs can be detected throughout the cortex. Investigators monitor ERPs by placing electrodes at areas like the midline (Fz, Cz, and Pz). A computer analyzes a subject's EEG responses to the same stimulus or task over many trials to subtract random EEG activity. ERPs always have the same waveform morphology. Their negative and positive peaks occur at regular intervals following the stimulus.Sensory evoked potentials are a subset of ERPs elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). They have a negative peak at 80-90 ms and a positive peak at about 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
Motor ERPs are detected over the primary motor cortex (precentral gyrus) during movement, and their amplitude is proportional to the force and rate of skeletal muscle contraction (Thompson & Thompson, 2016).
Slow cortical potentials modulate the excitability of associated neurons
Slow cortical potentials (SCPs) are gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials, and exclude event-related potentials (ERPS) (Andreassi, 2007).SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms (Steriade, 2005).
The movie is a 19-channel display of SCPs © John S. Anderson. Brighter colors represent higher SCP amplitudes. Negative SCPs drift down, and positive SCPs drift up. Negative SCPs are produced by the depolarization of apical dendrites and increase the probability of neuron firing. Positive SCPs are produced by the hyperpolarization of these dendrites and decrease the likelihood of neuron firing.
The contingent negative variation (CNV) is a steady, negative shift in potential (15 µV in young adults) detected at the vertex. This slow cortical potential may reflect expectancy, motivation, intention to act, or attention. The CNV appears 200-400 ms after a warning signal (S1), peaks within 400-900 ms, and sharply declines after a second stimulus that requires the performance of a response (S2). John Balven adapted the graphic below from Stern, Ray, and Quigley (2001).
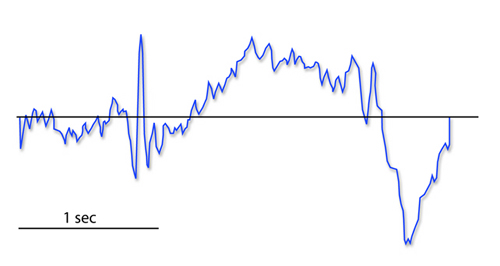
The readiness potential is a slow-rising, negative potential (10-15 µV) detected at the vertex before voluntary and spontaneous movement. This slow cortical potential precedes voluntary movement by 0.5 to 1 second and peaks when the subject responds. This potential is separate from the CNV. John Balven adapted the graphic below from Stern, Ray, and Quigley (2001).
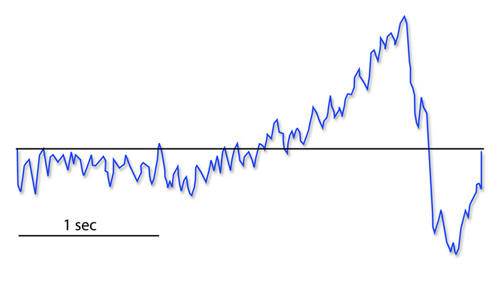
Movement-related potentials (MRPs) occur at 1 second as subjects prepare for unilateral voluntary movements. MRPs are distributed bilaterally with maximum amplitude at Cz. The supplementary motor area and primary motor and somatosensory cortices generate these potentials (Babiloni et al., 2002).
P300 and N400 ERPs are classified as long-latency potentials due to their extended latencies following stimulus onset.
The P300 potential is an event-related potential (ERP) with a 300-900-ms latency. The largest amplitude positive peaks are located over the parietal lobe. Researchers elicit the P300 potential by exposing subjects to an odd-ball stimulus, a meaningful stimulus that is different from others in a series (a colored playing card presented in a series of monochrome cards). The P300 potential may reflect an event’s subjective probability, meaning, and transmission of information. Research shows this is separate from the contingent negative variation (CNV) (Stern, Ray, & Quigley, 2001).
Shorter P300 latencies may reflect better allocation of attention, and researchers have measured longer P300 latencies in ADD than non-ADD samples. Experimental subjects show longer latencies when lying than when telling the truth (Farwell & Donchin, 1991; Thompson & Thompson, 2016).
The N400 potential is an event-related potential (ERP) elicited when we encounter semantic violations like ending a sentence with a semantically incongruent word ("The handsome prince married the beautiful fish"), or when the second word of a pair is unrelated to the first (BATTLE/GIRL). Warren and McIlvane (1998) speculate that the N400 potential is evoked whenever a general conceptual system that produces category judgments encounters a mismatch that violates equivalence relations. Halgren and colleagues (2002) consider it an index of the difficulty of semantic processing.
NEUROPLASTICITY (LTD AND LTP)
Neuroplasticity, the remodeling of neurons and neural networks with experience, is responsible for learning and memory. Memory storage involves the remodeling of neurons in terms of synaptic transmission, interneuron modulation, formation of new synapses, and rewiring of neural pathways (Bear, Connors, & Paradiso, 2020). Animal studies have shown that operant conditioning can induce astrogliogenesis (creation of new astrocytes) and neurogenesis (creation of new neurons) in structures like the medial prefrontal cortex and hippocampus (Rapanelli, Frick, & Zanutto, 2011).
The graphic is from synapse remodeling research by the Zuo Laboratory, MCD Biology, UCSC.
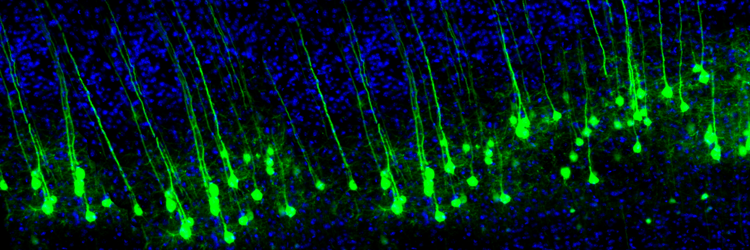
Neuroplasticity appears to involve a simple rule: when some synapses strengthen, adjacent synapses weaken to prevent overload due to increased input. A protein called Arc is crucial to this process (El-Boustani et al., 2018).
Neurofeedback, which involves the operant conditioning of CNS electrical activity, would be impossible without neuroplasticity. In neurofeedback, clients may learn to change the activity of local, regional, and global cortical resonant loops and the connectivity between brain regions (Collura, 2014; Thompson & Thompson, 2016).
To learn more about neuroplasticity, view the Khan Academy video Neuroplasticity.
Long-Term Depression and Long-term Potentiation
Two of the diverse processes involved in neuroplasticity are long-term depression and long-term potentiation.In long-term depression (LTD), synaptic transmission that coincides with slight depolarization of the postsynaptic neuron weakens a synapse due to pre- and postsynaptic changes. Relatively low-frequency stimulation of afferent neurons reduces the magnitude of their response to future stimulation
In long-term potentiation (LTP), synaptic transmission that coincides with strong depolarization of the postsynaptic neuron strengthens a synapse due to pre- and postsynaptic changes. Strong stimulation of afferent neurons results in a stable and persistent (weeks or more) increase in synaptic effectiveness. LTP involves diverse changes, including creating new synapses, enhancing previous synapses, and building new dendritic branches and spines (Breedlove & Watson, 2020).
To learn more, watch the Khan Academy video Long Term Potentiation and Synaptic Plasticity.
Glossary
40-Hz rhythm: gamma rhythm hypothesized to be associated with feature binding (linking an apple's color to its shape) and attributed to the neocortex and thalamocortical neurons.
acetylcholine: an amine neurotransmitter that binds to nicotinic and muscarinic ACh receptors.
acetylcholine esterase (AChE): the enzyme that deactivates ACh.
AChE-R: an abnormal form of acetylcholine esterase (AChE) may render dendrites with acetylcholine receptors more excitable when stressed.
action potential: a propagated electrical signal that usually starts at a neuron’s axon hillock and travels to presynaptic axon terminals.
adenylate cyclase: at a metabotropic receptor, an enzyme that transforms ATP into the second messenger cyclic AMP.
afferent: a neuron that transmits sensory information towards the central nervous system or from one region to another.
allocortex: cortex that contains three or four layers and is comprised of the olfactory system and hippocampus.
all-or-none law: once an action potential is triggered in an axon, it is propagated, without decrement, to the end of the axon. The amplitude of the action potential is unrelated to the intensity of the stimulus that triggers it.
alpha blocking: arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it entirely while increasing EEG power in the beta range.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons that are linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: regular bursts of alpha activity.
alpha-subunit: a subunit of a G protein associated with the neuron membrane that breaks away to activate enzymes within the neuron when a ligand binds to a metabotropic receptor.
amino acid neurotransmitters: the oldest family of transmitters. These molecules bind to ionotropic and metabotropic receptors, so they transmit information and modulate neuronal activity. In the brain, most synaptic communication is accomplished by glutamate (generally excitatory) and GABA (generally inhibitory).
AMPA (glutamate) receptors: ionotropic receptors which open sodium channels, depolarize the neuron's membrane (producing an EPSP), and dislodge a Mg+ ion that blocks an adjacent NMDA (glutamate) receptor's calcium channel. AMPA receptors are responsible for most activity at glutamatergic synapses.
amplitude: the energy or power contained within the EEG signal measured in microvolts or picowatts.
amygdala: the limbic system structure that participates in evaluating whether stimuli are salient (rewarding or threatening), establishing unconscious emotional memories, learning conditioned emotional responses, and producing anxiety and fear responses.
anion: a negative ion, for example, chloride (Cl-).
anterior: near or toward the front of the head, for example, the anterior cingulate.
anterior cingulate: a division of the prefrontal cortex that plays a vital role in attention and is activated during working memory. It mediates emotional and physical pain, and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components.
anterior commissure: a bundle of nerve fibers that crosses the midline and connects the left and right temporal lobes and the hippocampus and amygdala.
apical dendrite: a dendrite that arises from the top of the pyramid and extends vertically to layer 1 of the neocortex.
arousal: a process that combines alertness and wakefulness, produced by at least five neurotransmitters, including acetylcholine, histamine, hypocretin, norepinephrine, and serotonin.
aspinous (smooth) neurons: neurons without dendritic spines that are believed to be inhibitory.
astrocytes: star-shaped glial cells that communicate with and support neurons and help determine whether synapses will form.
asynchronous waves: neurons depolarize and hyperpolarize independently.
ATP: energy source for a neuron’s sodium-potassium transporters.
autoreceptors: metabotropic receptors that can be located on the membrane of any part of a neuron. They detect neurotransmitters the neuron releases, generate IPSPs that inhibit the neuron from reaching the excitation threshold, and regulate internal processes like transmitter synthesis and release through the second messenger system.
axoaxonic synapses: junctions between two axons that do not affect the generation of an action potential, only the amount of neurotransmitter distributed.
axodendritic synapses: junctions between axons and dendrites that determine whether the axon hillock will initiate an action potential.
axon: long, cylindrical structures that convey information from the soma to the terminal buttons. An axon also transports molecules in both directions along the outer surface of protein bundles called microtubules.
axon hillock: a swelling in the cell body where a neuron integrates the messages it has received from other neurons and decides whether to fire an action potential.
axon terminal: buds located on the ends of axon branches that form synapses and release neurochemicals to other neurons.
axonal varicosity: a swelling in an axon wall that releases neurotransmitters through the wall via volume transmission.
axoplasmic transport: the movement of molecules in both directions along the outer surface of protein bundles called microtubules.
basal dendrite: a dendrite that horizontally branches out from the 30 μm base of the pyramid through the layer where the neuron resides.
basal ganglia: forebrain structures consisting of an egg-shaped nucleus that contains the putamen and globus pallidus and a tail-shaped structure called the caudate, which together are responsible for the production of movement. The basal ganglia have also been implicated in obsessive-compulsive disorder, Parkinson’s disease, and Huntington’s chorea.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
bilateral synchronous slow waves: a pathological sign observed in drowsy children. When detected in alert adults, intermittent bursts of high amplitude slow waves may signify gray matter lesions in deep midline structures.
cation: positive ion, for example, sodium (Na+).
caudal: away from the front of the head.
cell body or soma: part of a neuron that contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
central nucleus of the amygdala: nucleus that orchestrates the nervous system's response to important stimuli by activating circuits in the brainstem (autonomic arousal) and the basal ganglia and periaqueductal gray (defensive behavior).
cerebral cortex: the layer of gray matter that covers the cerebral hemispheres. The cerebral cortex consists of gray matter and white matter.
chemical synapses: junctions between neurons that transmit molecules across gaps of less than 300 angstroms. Neurons use chemical synapses to produce short-duration (millisecond) and long-duration (seconds to hours) changes in the nervous system. Chemical synapses are capable of more extensive communication and initiating more diverse and long-lasting changes than electrical synapses.
classical routes for EEG activation: specific sensory pathways like the visual (retina to the visual cortex), auditory (cochlea to the auditory cortex), and somatosensory (chemoreceptors and mechanoreceptors to the somatosensory cortex) systems. Increased transmission of information through these pathways desynchronizes EEG activity in the cortical regions to which these afferent neurons project, as specialized circuits of neurons independently process this information.
commissures: axon tracts. The left and right hemispheres communicate using the corpus callosum, anterior commissure, and posterior commissure.
complex: a sequence of waves.
COMT: a degrading enzyme that only targets the catecholamines dopamine and norepinephrine.
contingent negative variation (CNV): a steady, negative shift in potential (15 microvolts in young adults) detected at the vertex. This slow cortical potential may reflect expectancy, motivation, intention to act, or attention. The CNV appears 200-400 ms after a warning signal (S1), peaks within 400-900 ms, and sharply declines after a second stimulus that requires the performance of a response (S2).
continuous irregular delta: slow waves produced by white matter lesions seen in disorders like multiple sclerosis.
contralateral: structures that are located on opposite sides of the body. For example, neurons in the left primary motor cortex control muscles on the right side of the body.
corpus callosum: the largest commissure that connects the left and right frontal, parietal, and occipital lobes.
corticothalamic network: a unified network that generates diverse types of brain rhythms grouped by slow cortical oscillations.
cyclic AMP: a second messenger that moves about the neuron, activating other enzymes. Protein kinase A, which controls the excitability of ion channels, is a crucial enzyme target of cyclic AMP. Cyclic AMP also travels to the nucleus, where it can regulate gene expression.
Dale's principle: incorrect view that a neuron can only release one neurotransmitter. They often release two to four.
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
dendrite: a branched structure designed to receive messages from other neurons via axodendritic synapses (junctions between axons and dendrites), determining whether the axon hillock will initiate an action potential.
dendritic spines: protrusions on the dendrite shaft where axons typically form axodendritic synapses.
dendrodendritic synapses: junctions between dendrites that communicate chemically across synapses and electrically across gap junctions.
depolarization: to make the membrane potential less negative by making the inside of the neuron less negative with respect to its outside.
diffusion: the distribution of molecules from areas of high concentration to low concentration.
diphasic wave: a wave that contains both a negative and positive deflection from the baseline.
dipole: the electrical field generated between the sink (where current enters the neuron ) and the source (place at the other end of the neuron where current leaves), which may be located anywhere along the dendrite.
dominant frequency: the EEG frequency with the most significant amplitude.
dopamine: a monoamine neurotransmitter exerts its postsynaptic effects on at least six receptors linked to G proteins. This means that dopamine functions as a neuromodulator. The two families include D1 (D1 and D5) and D2 (D2A, D2B, D3, and D4).
dorsal: toward the upper back or head.
dorsolateral prefrontal cortex: the left dorsolateral prefrontal cortex is concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals. The right dorsolateral prefrontal cortex organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
D-serine: a neurotransmitter that binds to the glycine site on the NMDA receptor to trigger calcium entry into a dendritic spine when glutamate binds to its site, resulting in a large, prolonged increase in intracellular calcium.
dual-action antidepressants: medications that activate 5-HT1 receptors to produce antidepressant and anxiolytic effects, while they blockade 5-HT2 (agitation, restlessness, and sexual dysfunction) and 5-HT3 (nausea, headache, and vomiting) receptors to minimize their side effects.
EEG activity: single wave or successive waves.
EEG power: the signal energy in the EEG spectrum. Most EEG power falls within the 0-20 Hz frequency range. EEG power is measured in microvolts or picowatts.
efferent: motoneuron that transmits information towards the periphery.
electrical synapse: symmetrical synapse where neurons communicate information bidirectionally across gap junctions between adjacent membranes using ions. Transmission across electrical synapses is instantaneous, compared with the 10-ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of distant neurons to synchronize their activity and simultaneously fire.
electroencephalogram (EEG): the voltage difference between at least two electrodes, where at least one electrode is located on the scalp or inside the brain. The EEG is a recording of EPSPs and IPSPs that occur primarily in dendrites in pyramidal cells located in macrocolumns, several millimeters in diameter, in the upper cortical layers.
electrostatic pressure: the attractive or repulsive force between ions that moves them from one region to another.
entorhinal cortex: a structure located in the caudal region of the temporal lobe and receives pre-processed sensory information from all modalities and reports on cognitive operations. The entorhinal cortex provides the main input to the hippocampus, and is involved in memory consolidation, spatial localization, and provides input into the septohippocampal system that may generate the 4-7 Hz theta rhythm.
enzymatic deactivation: the process in which an enzyme breaks a neurotransmitter apart into inactive fragments. For example, acetylcholine transmission is ended by the enzyme acetylcholine esterase (AChE). Deactivating enzymes located in the synaptic cleft degrade a neurotransmitter molecule when it detaches from its binding site.
evoked potential: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). An evoked potential has a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
excitatory postsynaptic potential (EPSP): a brief positive shift in a postsynaptic neuron's potential produced when neurotransmitters bind to receptors and cause positive sodium ions to enter the cell. An EPSP pushes the neuron towards the threshold of excitation when it can initiate an action potential.
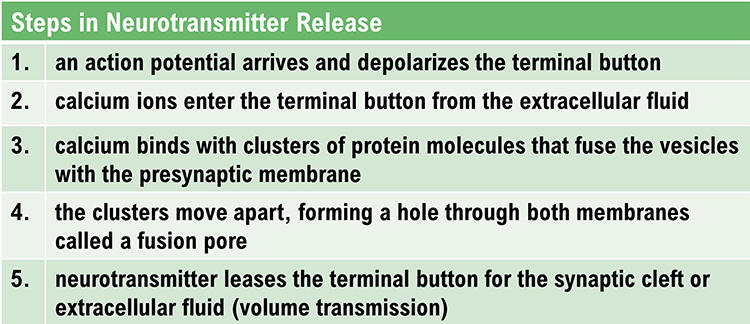
exocytosis: the process of neurotransmitter release. When an action potential arrives and depolarizes the terminal button, calcium ions enter the terminal button from the extracellular fluid. Calcium binds with clusters of protein molecules that connect the vesicles to the presynaptic membrane. The clusters move apart, forming a hole through both membranes called a fusion pore, and the neurotransmitter leaves the terminal button for the synaptic cleft or extracellular fluid.
exogenous ERP: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual).
explicit learning: behavioral changes that occur with our conscious awareness that require processing by the hippocampus.
extracellular dipole layers: macrocolumns of pyramidal cells, which lie parallel to the surface of the cortex, send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons.
extracellular fluid: the fluid surrounding a neuron.
fast cortical potentials: EEG rhythms that range from 0.5 Hz-100 Hz. The main frequency ranges include delta, theta, alpha, sensorimotor rhythm, and beta.
feature binding: the process of linking information to perceptual objects (linking an apple's color to its shape) that may involve the 40-Hz rhythm.
fissures: deep grooves, for example, the lateral fissure.
focal waves: EEG waves that are detected within a limited area of the scalp, cerebral cortex, or brain.
frequency: the number of cycles completed each second expressed in hertz (Hz).
frequency synchrony: when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
frontal lobes: the most anterior cortical lobes of the brain that are divided into the motor cortex, premotor cortex, and prefrontal cortex.
fusion pore: a hole through a vesicle and presynaptic membrane that allows neurotransmitter to leave the terminal button for the synaptic cleft or extracellular fluid.
G protein: a protein located inside a neuron’s membrane next to a metabotropic receptor that is activated when the receptor binds a ligand. An alpha-subunit of the G protein then breaks away to perform actions within the cell.
GABA: an amino acid that is often inhibitory and that may be the most important inhibitory neurotransmitter in the brain. There are several types of GABA receptors, each of which produces inhibition differently.
gamma rhythm: EEG activity frequencies above 30 or 35 Hz. Frequencies from 25-70 Hz are called low gamma, while those above 70 Hz represent high gamma.
gap junction: narrow space between two cells bridged by connexons (protein channels) that allow ions to travel between them rapidly.
generalized asynchronous slow waves: waves that are seen in sleepy children and those with elevated temperatures. These waves may indicate degenerative disease, dementia, encephalopathy, head injury, high fever, migraine, and Parkinson's disease in adults.
glial cells: nonneural cells that guide, insulate, and repair neurons and provide structural, nutritional, and information-processing support. Glial cells generate slow cortical potentials (SCPs). Glial cells include astrocytes, microglia, oligodendrocytes, radial glial cells, and Schwann cells.
glutamate: an amino acid that is often excitatory and that may be the primary excitatory neurotransmitter in the brain. Its receptors are found on the surface of almost all neurons. There are at least 13 different receptors for glutamate, 5 ionotropic and 8 metabotropic. Most presynaptic neurons in the brain excite postsynaptic neurons via ionotropic glutamate receptors in the postsynaptic membrane. Metabotropic glutamate receptors may play a regulatory function, either augmenting or suppressing the activation of ionotropic glutamate receptors.
glycine: an amino acid that is often inhibitory and has a binding site on the NMDA receptor.
gray matter: brain tissue that looks grayish brown and comprises cell bodies, dendrites, unmyelinated axons, glial cells, and capillaries.
gyrus: ridge of cortex demarcated by sulci or fissures, for example, the precentral gyrus.
hertz (Hz): the unit of frequency, an abbreviation for cycles per second.
hippocampus: a limbic structure located in the medial temporal lobe involved in 4-7 Hz theta activity, control of the endocrine system’s response to stressors, formation of explicit memories, and navigation. Cortisol binding to this structure disrupts these functions, interferes with creating new neurons, and harms and kills hippocampal neurons.
hubs: highly centralized nodes through which other node pairs communicate; hubs allow efficient communication.
hyperpolarize: a negative shift in membrane potential (the inside becomes more negative with respect to the outside) due to the loss of positive ions or gain of negative ions.
inhibitory postsynaptic potential (IPSP): a brief negative shift in a postsynaptic neuron's potential produced when cations like potassium leave a neuron or anions (negative ions) like chloride enter a neuron, which hyperpolarize the cell. An IPSP pushes the neuron away from its excitation threshold.
integration: the addition of EPSPs and IPSPs at the axon hillock. Neurons sum EPSPs and IPSPs over their surface in spatial integration and over milliseconds in temporal integration to raise the membrane from its resting potential to the excitation threshold. EPSPs and IPSPs last from 15-200 ms, while action potentials occur in 1-2 ms.
interneurons: neurons that receive input from and distribute output to other neurons. They have short processes and are confined to the central nervous system. They provide the integration required for decisions, learning and memory, perception, planning, and movement.
intracellular fluid: the watery cytoplasm contained within a neuron.
ion: a charged atom or molecule with a positive or negative charge. Positive ions are called cations, and negative ions are called anions.
ionotropic receptor: receptor protein that contains a binding site for a ligand and an ion channel that opens when the neurotransmitter attaches to this site.
ipsilateral: structures that are located on the same side of the body. For example, the left olfactory bulb distributes axons to the left hemisphere.
irregular waves: successive waves that constantly alter their shape and duration.
kappa rhythm: bursts of alpha or theta and is detected over the temporal lobes of subjects during cognitive activity.
lambda waves: saw-toothed transient waves from 20-50 μV in amplitude and 100-250 ms in duration detected over the occipital cortex during wakefulness. These positive deflections are time-locked to saccadic movements and observed during visual scanning, as during reading.
lateral: to the side, away from the center, as in the lateral geniculate nucleus.
lateral nucleus of the amygdala: a nucleus that processes sensory information and distributes it throughout the amygdala.
lateralized waves: waves that are primarily detected on one side of the scalp and that may indicate pathology.
Layers I-III: cortical layers that receive corticocortical afferent fibers that connect the left and right hemispheres.
Layer III: the cortical layer that is the primary source of corticocortical efferent fibers.
Layer IV: the cortical layer that is the primary destination of thalamocortical afferents and intra-hemispheric corticocortical afferents.
Layer V: the cortical layer that is the primary origin of efferent fibers that target subcortical structures that have motor functions.
Layer VI: the cortical layer that projects corticothalamic efferent fibers to the thalamus, which, together with the thalamocortical afferents, creates a dynamic and reciprocal relationship between these two structures.
left dorsolateral prefrontal cortex: the division of the prefrontal cortex concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals.
local field potential: the aggregate effect of the firing of the interconnected pyramidal neurons within the cortical columns plus additional mechanisms like glial cell modulation of the cortical electrical gradient.
local synchrony: synchrony that occurs when high-amplitude EEG signals are produced by the coordinated firing of cortical neurons.
localized slow waves: waves that may indicate a transient ischemic attack (TIA) or stroke, migraine, mild head injury, or tumors above the tentorium. Deep lesions result in bilateral or unilateral delta.
locus coeruleus: the noradrenergic branch of the ascending reticular activating system, which is responsible for vigilance. Subnormal norepinephrine transmission may contribute to ADHD.
long-latency potentials: potentials that have extended latencies following stimulus onset, for example, P300 and N400 ERPs.
long-term depression (LTD): a persistent decrease in synaptic strength following low-frequency stimulation.
long-term potentiation (LTP): a persistent increase in synaptic strength following high-frequency stimulation.
macrocolumns: circuits of cortical pyramidal neurons several millimeters in diameter that create extracellular dipole layers parallel to the surface of the cortex that send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons. Since the pyramidal neurons are all aligned with the cortical surface, the postsynaptic potentials at cells within the same macrocolumn add together. This summation occurs because they share the same charge and the macrocolumns fire synchronously.
medial: toward the center of the body, away from the side. For example, the medial geniculate nucleus.
medial prefrontal cortex: the division of the prefrontal cortex that integrates cognitive-affective information and helps control the hypothalamic–pituitary–adrenal (HPA) axis during emotional stress.
membrane potential: a neuron’s electrical charge created by a difference in ion distribution within and outside the neuron. A typical resting potential is about -70 mV (thousandths of a volt), since the inside of a resting axon is more negatively charged than the outside.
mesocortical neurons: dopaminergic neurons that project from the ventral tegmental area of the midbrain to the prefrontal cortex and excite prefrontal cortical neurons that control working memory, planning, and strategy preparation for problem-solving. Underactivity in this pathway is associated with the negative symptoms of schizophrenia-like attentional deficits.
metabotropic receptors: include all G protein-linked receptors located on neurons, including autoreceptors. Neurotransmitters that bind to G protein-linked receptors are often called neuromodulators. Metabotropic receptors, which indirectly control the cell's operations, expend energy, and produce slower, longer lasting, and more diverse changes than ionotropic receptors. Their effects can last several seconds, instead of milliseconds, because of the long-lived activity of G proteins and cyclic AMP.
microglia: microscopic glial cells that participate in the immune response.
microtubules: hollow cylindrical protein bundles that are involved in axoplasmic transport.
modulating effects: neuromodulators like the monoamines alter the performance of diffuse networks of target neurons by indirectly controlling cellular operations when they bind to metabotropic receptors.
module: a set of interconnected nodes in a neural network.
monoamine neurotransmitters: amine neurotransmitters that include dopamine, norepinephrine, epinephrine (catecholamines), and serotonin (indoleamine). These neurotransmitters are released using volume transmission and generally have modulating effects, altering the performance of diffuse networks of target neurons.
monoamine oxidase (MAO): an enzyme that degrades and inactivates the monoamine neurotransmitters dopamine, norepinephrine, and serotonin.
monoamine oxidase inhibitors (MAOIs): antidepressant drugs that interfere with MAO's breakdown of monoamines and increase monoamine availability to treat clinical depression.
monophasic wave: either a single negative (upward) or positive (downward) deflection from baseline.
motor cortex: the subdivision of the frontal lobe located in the precentral gyrus and guides fine motor coordination (like writing).
motor ERPs: event-related potentials detected over the primary motor cortex (precentral gyrus) during movement. Their amplitude is proportional to the force and rate of skeletal muscle contraction.
motor neurons: efferent neurons that convey commands to glands, muscles, and other neurons.
movement-related potentials (MRPs): slow cortical potentials that occur at 1 second as subjects prepare for unilateral voluntary movements. MRPs are distributed bilaterally with maximum amplitude at Cz. The supplementary motor area and primary motor and somatosensory cortices primarily generate these potentials.
mu rhythm: 7-11-Hz waves resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
multiple spike-and-slow-wave complex: multiple spikes associated with at least one slow wave.
muscarinic receptors: metabotropic ACh receptors that are stimulated by muscarine and blocked by atropine. Muscarinic receptors control smooth muscle and predominate in the CNS. In the CNS, muscarinic receptors help mediate learning, memory, attention, arousal, EEG, and postural control.
myelinated axons: axons that are insulated by myelin by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system.
N1-P2: a sensory event-related potential in the auditory cortex of the temporal cortex that reveals whether an uncommunicative person can hear a stimulus.
N400 potential: an event-related potential (ERP) elicited when we encounter semantic violations like ending a sentence with a semantically incongruent word ("The handsome prince married the beautiful fish"), or when the second word of a pair is unrelated to the first (BATTLE/GIRL).
negative SCPs: slow cortical potentials produced by glial cells that increase the probability of neuron firing.
neuroaxis: an imaginary line that runs centrally through the central nervous system (CNS) from the front of the prefrontal cortex to the base of the spinal cord.
neuromodulator: a neurochemical that modifies the effect of neurotransmitters through mechanisms like binding to metabotropic receptors.
neuron: a nerve cell that is the fundamental anatomical unit of the nervous system.
nicotinic ACh receptor: an ionotropic receptor that is stimulated by nicotine and blocked by curare. They are mainly found in the PNS on skeletal muscles. At CNS axoaxonic synapses, they produce presynaptic facilitation (increase neurotransmitter release). In the CNS, nicotinic receptors help regulate cortical blood flow, anxiety reduction, and decision-making.
nigrostriatal pathway: dopaminergic pathway from the substantia nigra to the basal ganglia (caudate nucleus and putamen) that controls movement. The nigrostriatal pathway is progressively destroyed in Parkinson’s disease.
nitric oxide: a gaseous retrograde transmitter that is involved in long-term potentiation (LTP).
NMDA (glutamate) receptors: ligand-gated and voltage-gated glutamate receptors that bind the glutamate agonist NMDA. NMDA receptors play an essential role in long-term potentiation (LTP).
node: vertex within a neural network.
nodes of Ranvier: gaps between myelinated axon segments where the axon membrane is exposed to extracellular fluid and action potentials are regenerated by sodium ion entry.
norepinephrine: a monoamine neurotransmitter that exerts postsynaptic effects at alpha and beta receptors, each with two subtypes. All norepinephrine receptors are G protein-linked. The cell bodies of the core noradrenergic system are located in the locus coeruleus, a nucleus found in the dorsal pons.
nucleus accumbens: a limbic structure that is a target of dopamine released by the mesolimbic pathway. The nucleus accumbens plays a critical role in the reinforcement of diverse activities, including ingestion of drugs like central nervous system stimulants.
occipital lobes: cortical lobes that are posterior to the parietal lobes. They process visual information from the eyes in collaboration with the frontal, parietal, and temporal lobes.
odd-ball stimulus: a meaningful stimulus that is different from others in a series used to elicit the P300 potential. For example, a colored playing card in a series of monochrome cards.
oligodendrocytes: glial cells that insulate adjacent axons within the brain and spinal cord of the central nervous system.
orbitofrontal cortex: the frontal lobe subdivision that is concerned with affective evaluation. It decodes the punishment and reward value of stimuli and helps inhibit inappropriate behavior. Phineas Gage's profound personality changes were produced by damage to this region.
orienting response: Pavlov’s "What is it?" reaction to stimuli like the sound of a vase crashing that includes (1) increased sensory sensitivity, (2) head (and ear) turning toward the stimulus, (3) increased muscle tone (reduced movement), (4) EEG desynchrony, (5) peripheral constriction and cephalic vasodilation, (6) a rise in skin conductance, (7) heart rate slowing, and (8) slower, deeper breathing.
P300 potential: an event-related potential (ERP) with a 300-900-ms latency. The largest amplitude positive peaks are located over the parietal lobe. The P300 potential may reflect an event’s subjective probability, meaning, and transmission of information.
paralimbic cortex: a transitional region between neocortex and allocortex.
parietal lobes: cortical lobes posterior to the frontal lobes that are divided into the primary somatosensory cortex (postcentral gyrus) and secondary somatosensory cortex. Their primary function is to process somatosensory information like pain and touch. The right posterior parietal lobe helps guide movements, locate objects in three-dimensional space, and create body boundaries.
phase: the degree to which the peaks and valleys of EEG waveforms coincide.
phase synchrony: synchrony when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
polarization: to make the membrane potential more negative by making the inside of the neuron more negative with respect to its outside.
polyphasic (multiphasic) wave: a wave that contains two or more deflections of opposite polarity from baseline.
positive SCPs: slow cortical potentials that are produced by glial cells that decrease the probability of neuron firing.
posterior: near or toward the back of the head.
posterior commissure: axon tracts located below the corpus callosum that connect the right and left diencephalon and mesencephalon.
precision: the number of voltage gradations or steps.
prefrontal cortex: the most anterior frontal lobe division and is subdivided into dorsolateral, medial, orbitofrontal, and anterior cingulate regions responsible for executive functions like attention and planning.
premotor cortex: the frontal lobe subdivision that is anterior to the motor cortex and helps program head, trunk, and limb movements.
presynaptic facilitation: a modulatory process in which a neuron increases the presynaptic neuron's neurotransmitter release by delivering a neurotransmitter that increases calcium ion entry into its terminal button.
presynaptic inhibition: a modulatory process in which a neuron decreases neurotransmitter release by reducing calcium ion entry.
primary somatosensory cortex (S1): the parietal lobe subdivision located at the postcentral gyrus that processes information about touch and pain.
protein kinase A: an intracellular enzyme that controls the excitability of ion channels and is a critical enzyme target of cyclic AMP.
raphe nuclei: serotonergic cell bodies in the midbrain, pons, and medulla give rise to most of the brain’s serotonergic neurons.
rate law: the principle that neurons represent the intensity of a stimulus by variation in the rate of axon firing.
readiness potential: slow-rising, negative potential (10-15 µV) detected at the vertex before voluntary and spontaneous movement. This slow cortical potential precedes voluntary movement by 0.5 to 1 second and peaks when the subject responds.
regular or monomorphic waves: successive waves with identical shapes. Regular waves may resemble sine waves (sinusoidal) or maybe arched (resembling wickets) or saw-toothed (asymmetrical and triangular).
resting potential: the membrane potential of a neuron when it is not influenced by messages from other neurons.
reticular formation: a network of 90 nuclei within the central brainstem from the lower medulla to the upper midbrain. The reticular formation sends axons to the spinal cord, thalamus, and cortex, contributing to diverse functions like neurological reflexes, muscle tone and movement, attention, arousal, and sleep.
reuptake: the primary method that neurons terminate the action of neurotransmitters. Reuptake transporters located in terminal buttons and astrocytes remove neurotransmitters from the synaptic cleft.
reward deficiency syndrome: Blum’s hypothesis that an abnormal form of the A1 allele is present in most severe alcoholics and results in defective D2 receptors. Reduced D2 receptor activity may reduce the activation of the nucleus accumbens and hypothalamus and result in dysphoria, drug craving, and compulsive drug-seeking and abuse.
right dorsolateral prefrontal cortex: the division of the prefrontal cortex that organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
rostral: toward the front of the head.
saltatory conduction: action potential conduction in myelinated axons in which action potentials jump from node to node for 200 times greater speed.
sampling rate: the number of measurements per second (Hz).
Schwann cells: glial cells that provide myelin for single PNS axons and facilitate axonal regeneration following damage.
secondary somatosensory cortex (S2): a region of the parietal lobe that receives somatosensory information from the primary somatosensory cortex (S1).
sensorimotor rhythm (SMR): EEG rhythm that ranges from 12-15 Hz and is located over the sensorimotor cortex (central sulcus). The waves are synchronous. The sensorimotor rhythm is associated with the inhibition of movement and reduced muscle tone. The SMR is generated by "ventrobasal relay cells in the thalamus and thalamocortical feedback loops."
sensorimotor system: in Sterman’s model, ascending pathways that convey information about touch and proprioception to the thalamus, the thalamus and its thalamic projections to the sensorimotor cortex, and the sensorimotor cortex, and its efferent fibers.
sensory event-related potentials (ERPs): event-related potentials evoked by external sensory stimuli (auditory, olfactory, somatosensory, and visual). These evoked potentials or exogenous ERPs have a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. These changes in brain activity in response to specific stimuli. ERPs can be detected throughout the cortex. Investigators monitor ERPs by placing electrodes at locations like the midline (Fz, Cz, and Pz). A computer analyzes a subject's EEG responses to the same stimulus or task over many trials to subtract random EEG activity. ERPs always have the same waveform morphology. Their negative and positive peaks occur at regular intervals following the stimulus.
sensory neurons: neurons specialized for sensory intake. They are called afferent because they transmit sensory information towards the central nervous system (brain and spinal cord).
septal nuclei: in Sieb’s model, when the prefrontal cortex receives information about high-priority environmental events, it signals cell bodies in the septum to induce a beta rhythm in the hippocampus to remove its inhibition of vigilance centers.
septohippocampal system: a subcortical circuit from the septum to hippocampus that contributes to 4-7 Hz theta activity.
septum: a limbic structure that contains several nuclei involved in emotion and addiction and control of aggressive behavior.
sharp transients: a sequence that contains several sharp waves.
sharp waves: waves that resemble spikes with a pointed peak with a longer 70-200-ms duration.
sink: a site where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, represented by -ve.
slow cortical potentials (SCPs): gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials. SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms.
sodium (Na+) ions: positive ions that enter a neuron during EPSPs and action potentials.
sodium-potassium transporters: pumps that are powered by ATP and that exchange three sodium for two potassium ions.
soma or cell body: the region of a neuron that contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
source: the place at the end of the neuron opposite of the sink where current leaves, represented by +ve. The extracellular area surrounding the source becomes electrically positive.
spatial summation: the addition of EPSPs and IPSPs over a neuron’s surface.
spike: a negative transient with a pointed peak at conventional paper speeds, 20-70-ms duration, and 40-100 μV amplitude; rare dendritic action potentials.
spike-and-slow-wave complex: a spike followed by a higher amplitude slow wave at 3 Hz. In an absence seizure, the amplitudes are very high (e.g., 160 μV).
spindle waves: waves that originate in the thalamus and occur during unconsciousness and stage II sleep.
spiny neurons: neurons with dendritic spines that are usually excitatory.
striatal: basal ganglia (caudate nucleus and putamen).
Stroop test: cognitive monitoring task where color and names conflict.
substantia nigra: midbrain structure that projects to the basal ganglia (caudate nucleus and putamen) to control movement and that is progressively destroyed in Parkinson’s disease.
sulcus: a shallow groove in the surface of the cerebral hemisphere, for example, the central sulcus.
synapse-associated polyribosome complexes (SPRCs): organelles with dendrites that can produce proteins that allow rapid remodeling of synapses. A polyribosome complex consists of several ribosomes bound to messenger RNA (mRNA). SPRCs represent one mechanism underlying synaptic plasticity.
synaptic cleft: 20-40-nm fluid-filled gap between presynaptic and postsynaptic structures.
synchronous: adverb meaning that groups of neurons depolarize and hyperpolarize simultaneously.
synchrony: the coordinated firing of pools of neurons. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
telencephalon: the frontal subdivision of the forebrain, including the cerebral cortex, basal ganglia, and limbic system.
temporal summation: the addition of EPSPs and IPSPs over time. Summation is more effective when postsynaptic potentials are generated more closely in time.
temporal lobes: lobes separated from the rest of the cortical lobes by the Sylvian fissure. The temporal lobes process hearing, smell, and taste information and help us understand spoken language and recognize visual objects and faces. The amygdala and hippocampus, which lie beneath the temporal cortex, play crucial roles in emotion, declarative, emotional, and working memory, and navigation.
terminal buttons: buds located on the ends of axon branches that form synapses and release neurochemicals to other neurons. They contain vesicles that store neurotransmitters for release when an action potential arrives. A terminal button’s presynaptic membrane may possess reuptake transporters that return neurotransmitters from the synapse or extracellular space for repackaging.
thalamus: forebrain structure above the hypothalamus that receives, filters, and distributes most sensory information. The thalamus contains neurons that can block or relay ascending sensory information. When these thalamic neurons rhythmically fire, this blocks the transmission of information to the cortex. When they depolarize in response to sensory information, this integrates and transmits this information to the cortex. Inputs to the thalamus determine whether these neurons block or relay sensory information.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
threshold of excitation: the membrane potential at which an axon initiates an action potential, nominally -40 mV.
traveling waves: EEG oscillations that move across the cortex that may mediate large-scale coordination of brain networks and support connectivity.
transient: a single wave or sequence of regular waves, called a complex, distinguishable from background EEG activity.
triphasic wave: a wave that contains three deflections from baseline.
unmyelinated axons: smaller-diameter axons without fatty insulation that conduct more slowly than myelinated axons.
+ve: the source is the place at the other end of the neuron where current leaves, and is represented by +ve.
-ve: a sink is where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, represented by -ve.
ventral: toward the base of the skull or front of the body.
ventral striatum: the olfactory tubercle and nucleus accumbens.
ventral tegmental area: the midbrain structure that distributes dopaminergic axons to the nucleus accumbens. Serotonin receptors on endorphin-releasing neurons in the hypothalamus may increase the activity of dopaminergic reward pathways by inhibiting the release of GABA at receptors on cell bodies of the ventral tegmental area neurons.
vigilance system: in Sterman’s model, a system that consists of both specific brainstem nuclei (e.g., locus coeruleus and raphe nuclei) and their diffuse connections with the thalamus and other subcortical structures, and the cortex. Several neurotransmitter systems mediate vigilance, including cholinergic/glutamatergic (reticular formation), noradrenergic (locus coeruleus), and serotonergic (raphe) neurons.
volume transmission: extrasynaptic neurotransmitter release from axonal varicosities, dendrites, and terminal buttons into the extracellular space. Monoamines like norepinephrine and serotonin are released outside the synaptic cleft.
waveform: the shape and form of an EEG signal.
white matter: the layer beneath the cortex that mainly consists of myelinated axons.
REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, how would you explain the relationship between local field potentials and the EEG? How does anatomy explain why the EEG is comprised of EPSPs and IPSPs instead of action potentials?
References
Advokat, C. D., & Comaty, J. E., & Julien, R. M. (2019). Julien's primer of drug action (15th ed.). Worth Publishers.
Aloisi, F. (2001). Immune function of microglia. Glia, 36, 165–179. https://doi.org/10.1002/glia.1106
Andreassi, J. L. (2007). Psychophysiology: Human behavior and physiological response (5th ed.). Lawrence Erlbaum and Associates, Inc.
Arnsten, A. F. (2006). Fundamentals of Attention-Deficit/Hyperactivity Disorder: Circuits and pathways. Journal of Clinical Psychiatry, 67 (Suppl. 8), 7-12.
Babiloni, C., Babiloni, F., Carducci, F., Cincotti, F., Del Percio, C., Hallett, M., Moretti, D. V., Romani, G. L., & Rossini, P. M. High resolution EEG of sensorimotor brain functions: Mapping ERPs or mu ERD? In R. C. Reisin, M. R. Nuwer, M. Hallett, & C. Medina (Eds.). Advances in Clinical Neurophysiology (Supplements to Clinical Neurophysiology Vol. 54). Elsevier Science B. V.
Bear, M. F., Connors, B. W., & Paradiso, M. A. (2020). Neuroscience: Exploring the brain (4th ed.). Jones & Bartlett Learning.
Breedlove, S. M., & Watson, N. V. (2020). Behavioral neuroscience (9th ed.). Sinauer Associates, Inc.
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature, 10, 186-198. https://doi.org/10.1038/nrn2575
Cameron, H. A., & Dayer, A. G. (2008). New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry, 63(7), 650-655. https://dx.doi.org/10.1016%2Fj.biopsych.2007.09.023
Carlson, N. R., & Birkett, M. A. (2021). Physiology of behavior (13th ed.). Pearson.
Chan, C. Y., Ke, D. S., & Chen, J. Y. (2009). Essential fatty acids and human brain. Acta Neurol Taiwan, 18(4), 231-241. PMID: 20329590
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Costanzo, R. M. (1991). Regeneration of olfactory receptor cells. CIBA Found Symp, 160, 233-242. https://doi.org/10.1002/9780470514122.ch12
Creuzfeldt, O. D. (1995). Cortex cerebri. Oxford University Press.
Damasio, A. (2010). Self comes to mind. Pantheon Books.
deCharms, R. C., Fumiko, M., Glover, G. H., Ludlow, D., Pauly, J. M., Soneji, D., Gabrieli, J. D. E., & Mackey, S. C. (2005). Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences, 102(51), 18626-18631. https://doi.org/10.1073/pnas.0505210102
DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci, 13(7), 281-285. https://doi.org/10.1016/0166-2236(90)90110-v
Demos, J. N. (2019). Getting started with neurofeedback. (2nd ed.). W. W. Norton & Company.
Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302, 290-292. https://doi.org/10.1126/science.1089134
El-Boustani, S., Ip, J., Breton-Provencher, V., Knott, G., Okuno, H., Bito, H., & Sur, M. (2018). Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science, 360(6395), 1349-1354. https://doi.org/10.1126/science.aao0862
Enticott, P. G., Kennedy, H. A., Rinehart, N. J., Tonge, B. J., Bradshaw, J. L., Taffe, J. R., Daskalakis, Z. J., & Fitzgerald, P. B. (2012). Mirror neuron activity associated with social Impairments but not age in Autism Spectrum Disorder. Biol Psychiatry, 71(5), 427-433. https://doi.org/10.1016/j.biopsych.2011.09.001
Evans, J. R., & Abarbanel, A. (1999). Introduction to quantitative EEG and neurofeedback. San Diego: Academic Press.
Farwell, L. A., & Donchin, E. (1991). The truth will out: Interrogative polygraphy (“lie detection”) with event-related brain potentials. Psychophysiology, 28, 531–547. https://doi.org/10.1111/j.1469-8986.1991.tb01990.x
Fox, S. I., & Rompolski, K. (2022). Human physiology (16th ed.). McGraw-Hill.
Garrett, B. (2003). Brain and behavior. Thompson/Wadsworth.
Hansson, E., & Ronnback, L. (2003.) Glial neuronal signaling in the central nervous system. FASEB J, 17, 341-348. https://doi.org/10.1096/fj.02-0429rev
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Kalat, J. W. (2019). Biological psychology (13th ed.). Cengage Learning.
Kennerley, S. W., Behrens, T. E., & Wallis, J. D. (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci, 14(12), 1581-1589. doi:10.1038/nn.2961
Kitamura, T., Saitoh, Y., Takashima, N., Murayama, A., Niibori, A., Ageta, H., . . . Inokuchi, K. (2009). Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell, 139(4), 814-827. https://doi.org/10.1016/j.cell.2009.10.020
Klein, S. B., & Thorne, B. M. (2007). Biological psychology. New York: Worth Publishers. Kropotov, J. D. (2009). Quantitative EEG, event-related potentials and neurotherapy. Academic Press.
Landisman, C. E., & Connors, B. W. (2005). Long-term modulation of electrical synapses in the mamillimeteralian thalamus. Science, 310(5755), 1809-1813. https://doi.org/10.1126/science.1114655
Meshorer et al. (2002). Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science, 295(5554), 508-512. https://doi.org/10.1126/science.1066752
Molenberghs, P., Cunnington, R., & Mattingley, J. B. (2011). Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev, 36(1), 341-349. https://doi.org/10.1016/j.neubiorev.2011.07.004
Munro, C. A., McCaul, M. E., Wong, D. F., Oswald, L. M., Zhou, Y., Brasic, J., Kuwabara, H., Kumar, A., Alexander, M., Ye, W., & Wand, G. S. (2006). Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry, 59(10), 966-974. https://doi.org/10.1016/j.biopsych.2006.01.008
Nash, J. M. (2011). The gift of mimicry. Your brain: A user's guide. Time.
Radley, J. J., Arias, C. M., & Sawchenko, P. E. (2006). Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. The Journal of Neuroscience, 26(50), 12967-12976. https://doi.org/10.1523/JNEUROSCI.4297-06.2006
Raichle, M. E., & Gusnard, D. A. (2002). Appraising the brain's energy budget. PNAS, 99(16), 10237-10299. https://doi.org/www.pnas.orgcgidoi10.1073pnas.172399499
Rapanelli, M., Frick, L. R., & Zanutto, B. S. (2011). Learning an operant conditioning task differentially induces gliogenesis in the medial prefrontal cortex and neurogenesis in the hippocampus. PLoS ONE, 6(2), e14713. https://doi.org/10.1371/journal.pone.0014713
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L., & von Stein, A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci, 95(12), 7092-7096. https://doi.org/10.1073/pnas.95.12.7092
Schacter, D. L. (1977). EEG theta waves and psychological phenomena: A review and analysis. Biological Psychology, 5, 47-82. https://doi.org/10.1016/0301-0511(77)90028-x
M. S. Schwartz, & F. Andrasik (Eds.). (2003). Biofeedback: A practitioner's guide (3rd ed.). The Guilford Press.
Stahl, S. M. (2008). Stahl’s essential psychopharmacology: Neuroscientific basis and practical applications (3rd ed.). Cambridge University Press.
Steriade, M. (2005). Cellular substrates of brain rhythms. In E. Niedermeyer, & F. Lopes da Silva (Eds.). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Sterman, M. B. (2000). EEG markers for attention deficit disorder: Pharmacological and neurofeedback applications. Child Study Journal, 30(1), 1-24.
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Streit, W. J. (2006). Microglial senescence: Does the brain's immune system have an expiration date? Trends in Neurosciences, 29(9), 506–510. https://doi.org/10.1016/j.tins.2006.07.001
Thompson, M., & Thompson, L. (2016). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Thompson, M., & Thompson, L. (2009). Asperger’s syndrome intervention: Combining neurofeedback, biofeedback, and metacognition. In T. H. Budzynski, H. K. Budzynski, J. R. Evans, & A. Abarbanel (Eds.). Introduction to quantitative EEG and neurofeedback (2nd ed.). Academic Press.
Voytek, B. (2013). Brain metrics: How measuring brain biology can explain the phenomena of mind. Scitable by Nature Education.
Warren, A. M., & McIlvane, W. J. (1998). Stimulus equivalence and the N400 effect. Poster presented at the 1998 Annual Meeting of the Cognitive Neuroscience Society in San Francisco, CA.
Wilson, J. (2003). Biological foundations of human behavior. Wadsworth/Thompson Learning.
Wilson, V. E., Thompson, M., Thompson, L., Thompson, J., Fallahpour, K., & Linden, M. K. (2011). Introduction to biofeedback (Neurofeedback). In B. W. Strack, M. K. Linden, & V. S. Wilson (Eds.). Biofeedback & neurofeedback applications in sport psychology. Association for Applied Psychophysiology and Biofeedback.
Winn, P. (2001). (Ed.), Dictionary of biological psychology. Routledge.
Xu, T., Yu, X., Perlik, A., Tobin, W., Zweig, J., Tennant, K., Jones, T., & Zuo, Y. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature, 462(7275), 915-919. https://doi.org/10.1038/nature08389
Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., Poo, M., & Duan, S. (2003). Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proceedings of the National Academy of Sciences of the United States of America, 100(25), 15194-15199. https://doi.org10.1073/pnas.2431073100
Zhang, H., Watrous, A., Patel, A., & Jacobs, J. (2018). Theta and alpha oscillations are traveling waves in the human neocortex. Neuron. https://doi.org/10.1016/j.neuron.2018.05.019
B. NEUROANATOMY
The simplest way to divide the cortex is into the frontal and posterior cortex. The frontal cortex (frontal lobe) specializes in action, ranging from cognition, emotion, and autonomic control to movements and speech. The posterior cortex (parietal, temporal, and occipital lobes) is concerned with perception and memory. The frontal and posterior cortex, along with subcortical structures and the peripheral nervous system, provide the hierarchically arranged feedback loops that allow us to interact with our environment to achieve goals successfully.
The prefrontal cortex (PFC) (cortex rostral to the motor association cortex) directs the cognitive and emotional processes, called perception-action cycles, that adapt (and preadapt) us to our environment. The PFC predicts and creates the future. Working in cooperation with networked brain structures, the PFC marshals its executive functions of planning, attention, working memory, and decision making to develop innovative and sophisticated actions to pursue future goals (Fuster, 2015).
The nervous system uses bottom-up (feedforward) and top-down (feedback) processing to maintain homeostasis. The interconnectedness of neural networks is best illustrated by the relationship between the thalamus and cortex. Ascending thalamocortical neurons distribute sensory information to appropriate cortical (and subcortical) regions, and descending corticothalamic neurons convey instructions to the thalamus. The nervous system generates EEG activity, ranging from DC potentials to beta-gamma rhythms, using multiple generators that operate as neuroscientist William Calvin's "cerebral symphony." Graphic © adike/Shutterstock.com.


This unit covers Basic Neuroanatomy of Ascending Sensory Pathways to the Cortex, Thalamic, Cortical, and Subcortical Generators of the EEG, General Cortical and Subcortical Anatomy, Major Functions of Cortical Lobes and Major Subcortical Structures and Brodmann Areas, and Overview of Connectivity, Phase, and Coherence Concepts Related to EEG Networks and Tracts.
BASIC ANATOMY OF ASCENDING SENSORY PATHWAYS TO THE CORTEX
Sensory input produced by activities like reading a novel and listening to music can desynchronize cortical activity resulting in lower-amplitude, higher-frequency EEG waveforms (Neumann, Strehl, & Birbaumer, 2003). Arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it, a phenomenon called alpha blocking, while increasing EEG power in the beta range (Andreassi, 2007).
The classical routes for EEG activation consist of ascending sensory pathways that distribute information to specialized thalamic nuclei and then project the results of thalamic processing to the appropriate cortical regions. The exception to this rule is olfaction (smell), which goes directly to the primary olfactory cortex.
The old-school view is that these ascending sensory pathways exercise bottom-up control of perception as feedforward circuits. This overlooks the fact that ten times more cortical efferent neurons target the sensory thalamus as thalamic afferent neurons project to the cortex.
The new-school view is that there are extensive interconnections between the thalamus and cortex, which permit a degree of top-down cortical control over perception (Kandel et al., 2021).
We will examine the visual, auditory, and somatosensory systems to better understand the ascending sensory pathways to the cortex.
Visual System
Retinal ganglion cells, which comprise the optic nerves, ascend to the midline optic chiasm where the two optic nerves meet. Here, temporal axons (toward the side of the head) continue as part of their own side's optic tract, and the nasal halves cross over to join the opposite side's optic tract. Most optic tract axons project information to the lateral geniculate nucleus of the thalamus.While the lateral geniculate nucleus (LGN) relays visual information to the cortex, brainstem, and cortical neurons modulate its activity. Brainstem neurons that mediate alertness and attention can adjust the LGN's response to visual input. Moreover, the cortex can exert a top-down selection of visual input to increase attention to a salient region of the visual field at the expense of others (Bear, Connors, & Paradiso, 2020).
LGN neurons form the optic radiations and project to the primary visual cortex (V1) in the occipital lobe in cortical layer IV. A minority of retinal ganglion cell axons target the dorsal midbrain superior colliculus, which is concerned with visual gaze direction and attention to selected visual objects (Breedlove & Watson, 2020).
The cortex contains many specialist regions to process visual properties like color, shape, location, motion, and orientation. Evolution has organized these visual areas into dorsal and ventral streams that begin in the primary visual cortex. The dorsal stream, which projects from V1 to the parietal lobe, helps us localize objects and guide movements. There are neurons with visual and motor properties in the adjacent motor association cortex, called mirror neurons. Networks of mirror neurons may play a role in learning how to perform actions by observing others' movements, understanding others' actions and intentions, and empathy
The lower ventral stream, which projects to inferior temporal and frontal areas, allows us to identify objects and faces.
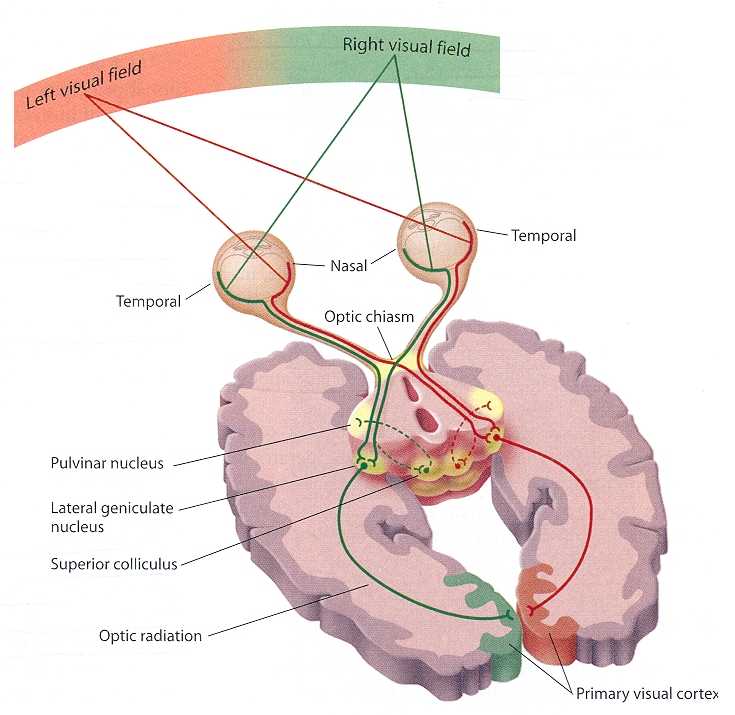
Auditory System
The cochlea's inner hair cells within the organ of Corti send 30,000 to 50,000 auditory fibers to several destinations: the midbrain superior olivary nuclei and inferior colliculi, and the medial geniculate nucleus of the thalamus. The superior olivary nuclei process binaural (two-ear) information to localize sound. All ascending auditory neurons innervate the inferior colliculi, some via intermediate relays.The inferior colliculi integrate information about spatial localization and multiple sensory modalities, including somatosensory information. They project to the thalamus' medial geniculate nucleus (MGN), which projects to several cortical auditory areas using two separate pathways. The MGN mainly relays frequency, amplitude, and binaural information to the auditory cortex in the temporal lobe. Graphic courtesy of www.cochlea.eu.
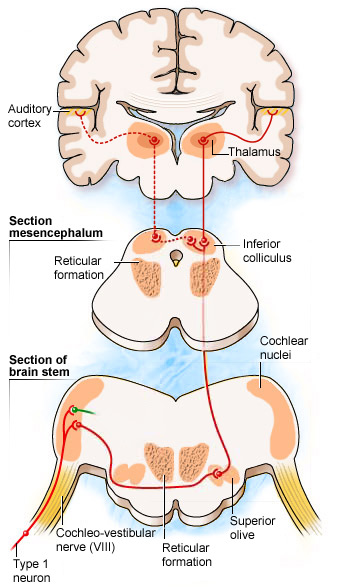
The auditory cortex processes auditory information within dorsal and ventral streams. The dorsal stream, which extends to the parietal lobe, helps us spatially localize sounds. The lower ventral stream, which projects to the temporal lobe, appears to analyze sound components, perhaps including speech sounds (Breedlove & Watson, 2020).
As with the visual pathways, the auditory system involves extensive feedback. Neurons in the brainstem innervate the outer hair cells that adjust the sensitivity of the basilar membrane within the organ of Corti to specific frequencies. Also, auditory cortex axons innervate both the inferior colliculi and MGN to exercise top-down control (Bear, Connors, & Paradiso, 2020).
Somatosensory System
The somatosensory system employs specialized receptors to perceive itch, pain, temperature, and touch. For touch, the axon of a unipolar neuron enters the dorsal horn of the spinal cord and synapses with a dorsal column neuron in the medulla. Axons from this neuron decussate (cross the midline) and innervate the thalmus' ventral posterior nucleus (VPN). The VPN, in turn, distributes this information to the primary somatosensory cortex (S1). While each hemisphere's S1 maps touch information from the opposite side of the body, the secondary somatosensory cortex (S2) maps both sides. The maps are overlaid so that the left and right arms are represented in the same region of the body surface map (Breedlove & Watson, 2020). Graphic courtesy of www.rci.rutgers.edu.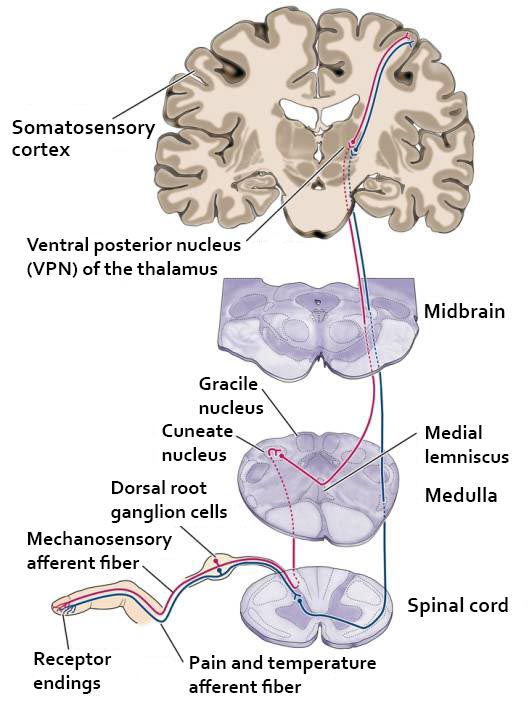
As with the visual and auditory systems, the ascending pathways do far more than relay information. These networks process and alter sensory information at each successive synapse. The cortex exercises top-down control over neurons in the dorsal column and VPN to dynamically adjust cortical inputs (Bear, Connors, & Paradiso, 2020).
THALAMIC, CORTICAL, AND SUBCORTICAL GENERATORS OF THE EEG
Thalamic Generators
Anderson and Anderson (1968) advanced the facultative pacemaker theory that thalamic neurons activate cortical neurons and thalamic inhibitory interneurons via recurrent collaterals. While these thalamocortical neurons only excite a limited number of cortical neurons, thalamic interneurons inhibit a large pool of thalamocortical relay neurons. When the inhibition ends after one-tenth of a second, the thalamocortical neurons experience rebound excitation. This synchronized depolarization excites both cortical neurons and thalamic inhibitory interneurons, which will inhibit a more extensive pool of thalamocortical relay neurons and initiate another cycle of excitation and inhibition that produces EEG rhythms (Fisch, 1999). Graphic courtesy of Zachary Barry and featured in Wikipedia's article Recurrent ThalamoCortical Resonance.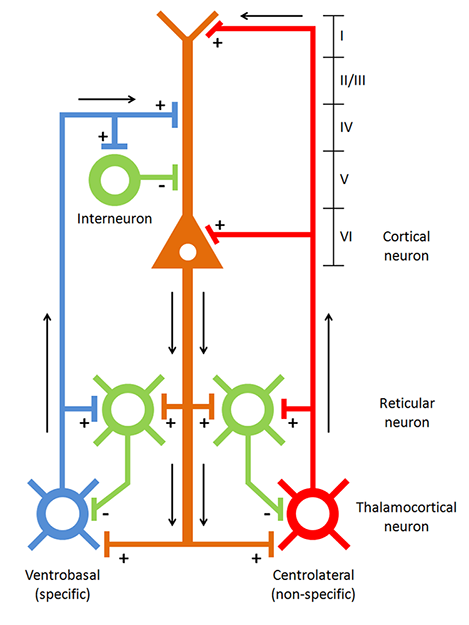
Caption: Thalamocortical circuit diagram depicting specific/sensory and non-specific intralaminar thalamocortical systems.
The networking of excitatory and inhibitory thalamic neurons imposes a group rhythm on its members that is transmitted to cortical macrocolumns by thalamocortical neurons (Bear, Connors, & Paradiso, 2020).
The nucleus reticularis of the thalamus may function as a pacemaker by releasing the inhibitory transmitter GABA at synapses with thalamocortical neurons. These neurons depolarize cortical neurons and thalamic inhibitory interneurons via burst discharges when their inhibition ends.
As discussed in the Neurophysiology unit, oscillatory activity may involve an interaction between thalamocortical relay neurons (TCR), nucleus reticularis neurons (RE), and interneurons. These interactions are mediated by diverse neurotransmitters, including acetylcholine and GABA.

The thalamus is the dominant pacemaker for rhythmic EEG activity, including theta (3-8 Hz), alpha (8-12 Hz), and SMR (13-15 Hz) (Amzica & Lopes da Silva, 2018).
Cortical Generators
The cerebral cortex (gray matter) consists of neuronal cell bodies, glial cells, and blood vessels. White matter lies beneath the neocortex, which consists of myelinated nerves, nonmyelinated fibers, and glial cells. The EEG mainly originates from pyramidal neurons in layers 3, 5, and 6 of gray matter that is approximately 5-mm thick. Check out the Blausen Cerebrum: Gray Matter and White Matter animation.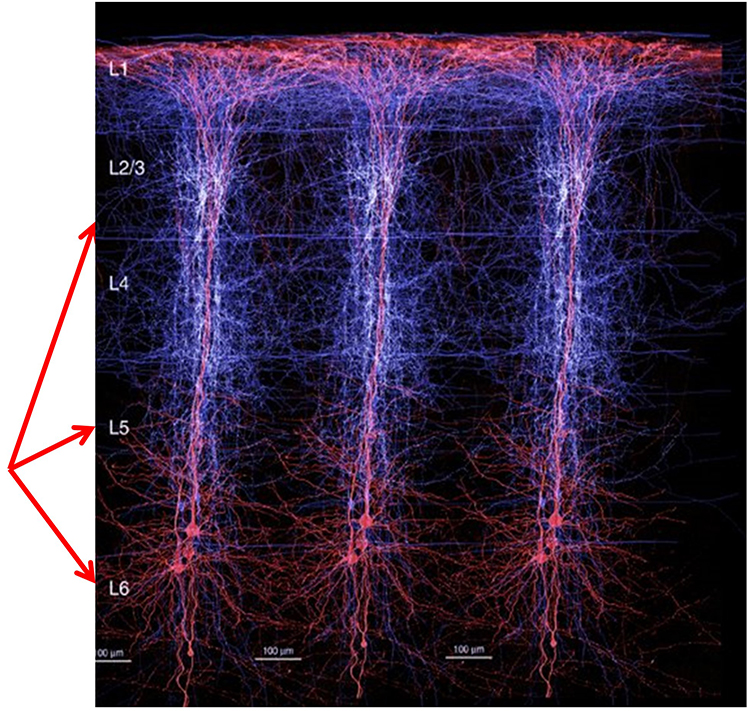
Vertical cortical macrocolumns contain hundreds of pyramidal neurons and supporting stellate and basket cells (Thompson & Thompson, 2016). Each pyramidal neuron may receive more than 100,000 synapses. These macrocolumns are positioned side by side and perpendicular to the cortical surface. Since neighboring macrocolumns often receive the same afferent messages, this increases the probability that they will fire together and generate a potential that we can detect from the scalp. A reliable scalp EEG requires a minimum of 6 cm² of synchronized cortex (Dyro, 1989).
Although thalamic pacemakers generate EEG rhythms, resonant loops between cortical macrocolumns may be another source (Traub et al., 1989). Over 97 percent of the conversations within the brain are cortical-to-cortical (Thompson & Thompson, 2016). This communication is primarily confined within the same hemisphere. A resonant loop develops when macrocolumns that share afferent input fire synchronously to generate an electrical potential. The distance between the cortical macrocolumns that participate in a resonant loop is one determinant of EEG frequency. The closer the macrocolumns in a resonant loop, the higher the frequency they can generate (Lubar, 1997).
The graphic below illustrates thalamocortical and cortical-cortical EEG sources.

There are three types of resonant loops driven by afferent input or thalamic pacemakers: local, regional, and global. Local loops couple neighboring macrocolumns and may generate frequencies above 30 Hz in the high-beta and gamma ranges. Regional loops couple macrocolumns separated by several centimeters and may produce alpha and beta rhythms. Finally, global loops couple macrocolumns as distant as 7 cm (for example, between the frontal and parietal lobes) and may create delta and theta rhythms.
While only 3 percent of these linkages are thalamocortical, they greatly influence the EEG by subcortically connecting distant cortical regions and producing most synchronous activity (Steriade, 1990). Lubar (1997) proposed a violin analogy where the thalamic pacemakers that fire at varying frequencies are the strings, and the resonant loops that introduce different time delays are the instrument's resonant cavity. Graphic of circuits that contribute to the EEG by Hindriks and van Putten © 2013 NeuroImage.
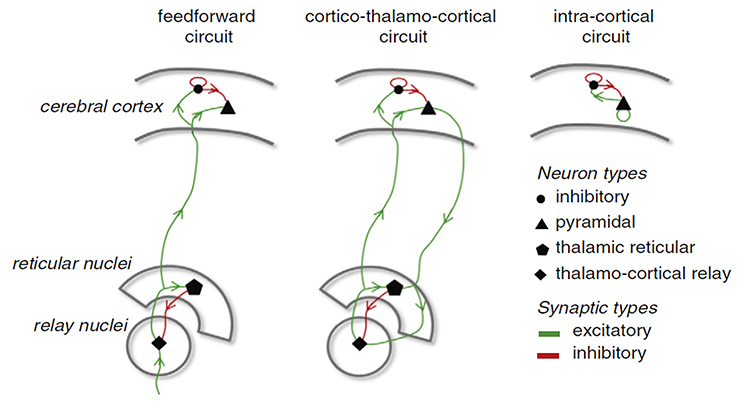
Spindling is a synaptically generated oscillation in a circuit that includes the reticular nuclei (Steriade, 2005). The video of alpha spindling © John S. Anderson.
Different spindle frequencies are due to corresponding durations of thalamocortical neuron hyperpolarization. For example, longer hyperpolarizations associated with EEG synchronized states produce 7-Hz or lower-frequency spindles. In contrast, relatively short hyperpolarizations result in 14 Hz spindles (Steriade, 2005).
The thalamocortical model © 2008 National Academy of Sciences from Izhikevich and Edelman's Large-scale model of mammalian thalamocortical systems article in the Proceedings of the National Academy of Sciences of the United States of America.
Caption: The researchers used diffusion tensor imaging to illustrate the targets of white-matter fibers.
The electrical potentials generated by the thalamus can volume conduct near the speed of light through cerebrospinal fluid (CSF), brain tissue, the skull, and the scalp so that nearly identical waveforms can simultaneously appear at distant sites (Fisch, 1999; Thompson & Thompson, 2016).The Locus Coeruleus Inhibits Thalamic Alpha Generators
When we are inattentive, thalamic pacemakers generate the alpha rhythm. When we need to focus attention, we activate the brainstem noradrenergic locus coeruleus. The increased release of norepinephrine by this 15-millimeter network focuses attention and abolishes alpha oscillations. The locus coeruleus enhances the brain's sensory information processing by suppressing thalamic alpha generators. This may be an underlying mechanism of the phenomenon of alpha blocking. Although researchers cannot noninvasively monitor locus coeruleus activity in human participants, it is correlated with pupil dilation. In human studies, the greater the alpha blocking response and pupil dilation, the better the performance on demanding attention tasks (Dahl et al., 2020; Dahl et al., 2022). Graphic © Vasilisa Tsoy/Shutterstock.The alpha rhythm is not a cause but a sign that incoming stimulation is too weak to overcome inhibition by the reticular nucleus. EEG activity is not causal and reflects network activity that has already occurred.

Additional Subcortical Generators
Ascending projections from the basal forebrain, reticular formation, locus coeruleus, and raphe systems disrupt brain rhythms.These neurons receive information from most sensory systems and cortical regions and directly desynchronize the EEG through synapses on cortical neurons and indirectly through innervation of thalamic pacemakers. Desynchronization shifts pyramidal neurons from burst firing to more continuous firing or the generation of single spikes (Fisch, 1999).The cholinergic basal forebrain, located in the ventral frontal lobe and anterior hypothalamus, influences cerebral blood flow and cognitive activity. Graphic courtesy of Newman et al. (2012), Cholinergic modulation of cognitive processing: Insights drawn from computation models.
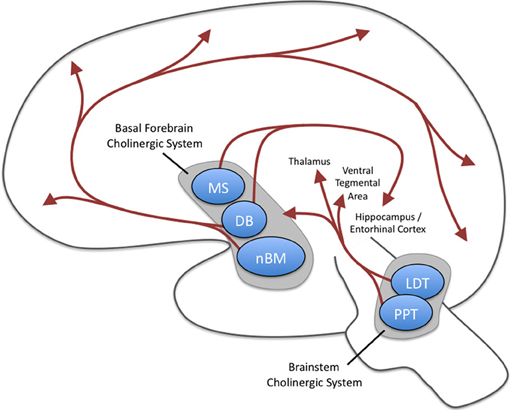
The reticular activating system (RAS) includes a network of 90 nuclei within the central brainstem from the lower medulla through the thalamus that activates the brain to promote attention, consciousness, and wakefulness. This network receives input from ascending sensory tracts (auditory, olfactory, somatosensory, and visual systems). The RAS projects to the thalamus and diffusely to the cortex. The RAS also has diffuse cortical projections that bypass the thalamus.

The noradrenergic brainstem locus coeruleus system, which projects to the thalamus, limbic system, and cerebral cortex, also contributes to wakefulness and vigilance for salient stimuli.
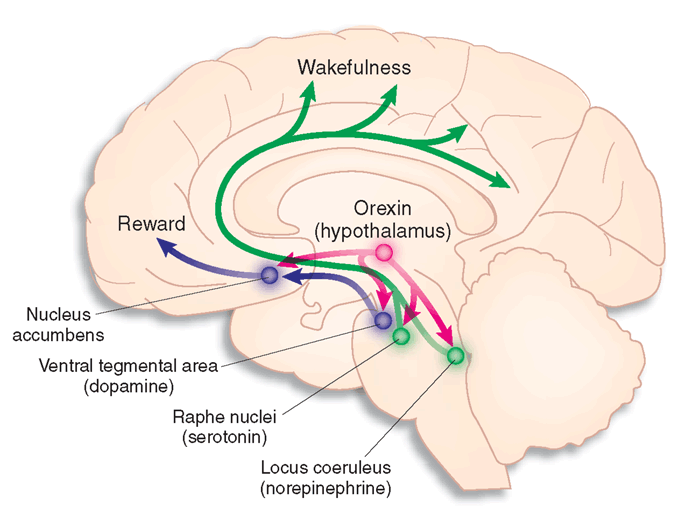
Finally, the serotonergic raphe system is a midline network of cell bodies within the brainstem and midbrain that may influence alertness and vigilance through reciprocal connections with the suprachiasmatic nucleus of the hypothalamus (Monti & Jantos, 2008).
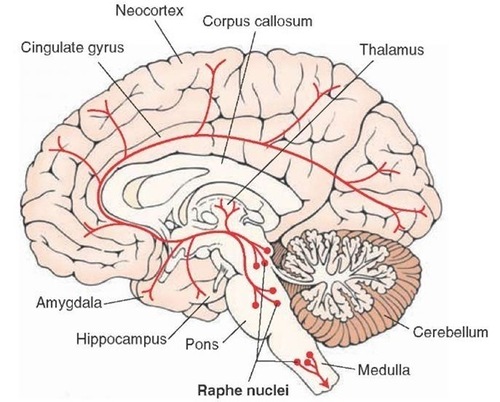
Cortical and Subcortical Generators of Specific EEG Rhythms
Slow Cortical Potentials (0-1 Hz)
Slow cortical potentials (SCPs) have been identified in cortical neurons, the thalamus, and glial cells. Cortical neurons in layers II to VI generate slow oscillations when the thalamus is removed or when cortical tissue is studied in vitro (in an artificial environment) or in vivo (within a living organism). Thalamic reticular neurons exhibit similar slow spontaneous oscillations when studied in vitro, and synchronized intracortical oscillations may depend on a corticothalamic network that targets these thalamic neurons.Glial cells generate slow SCPs when they burn sugar, producing negatively charged bicarbonate ions. Unlike EEG rhythms like delta, SCPs are not the summation of dendritic potentials. SCPs are associated with glial cells and gap junctions. Glial cells chemically communicate among themselves and with neurons. The slow oscillations of glial cells may influence the timing of neuronal firing through their control of potassium ion outflow (Steriade, 2005). These slow oscillations appear to organize the generation of other brain rhythms.
"The concept of a unified corticothalamic network that generates diverse types of brain rhythms grouped by the cortical slow oscillation (Steriade, 2001a,b) is supported by EEG studies in humans" (Molle et al., 2002).
Caton (1875) observed that the cortex's direct current baseline becomes negative whenever it is more active. The voltage gradients range from 150-200 μV. Underlying "tone" or valence factors determine the firing characteristics of neurons within a network. When SCPs are more positive, there is reduced firing of cortical neurons due to hypopolarization. When SCPs are more negative, there is increased firing due to depolarization.
The following 19-channel BioTrace+ /NeXus-32 display of 0.1-1 Hz SCP activity © John S. Anderson.
Perspective on Fast Cortical Potentials
EEG "bands" are somewhat arbitrary ranges of frequencies that have evolved from observation and usage. The following BioTrace+ /NeXus-32 video of raw and spectral EEG displays © John S. Anderson. Frequency is plotted along the horizontal axis, and amplitude is shown on the vertical axis.While frequency band labels are helpful descriptors, they can also be misleading. Classification of an EEG rhythm is based on context (measurement conditions and EEG activity during the specific epoch), frequency, and waveform morphology.
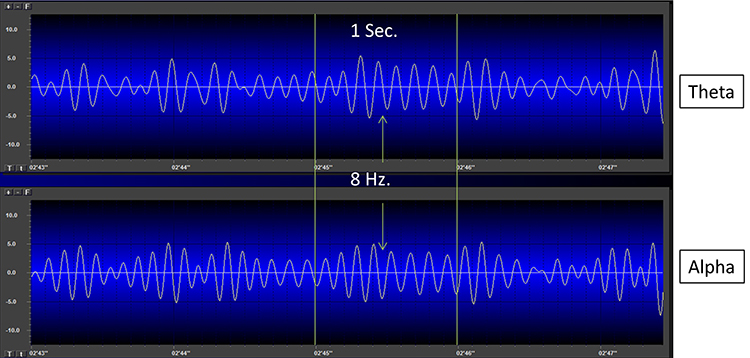
The process of up-training or down-training signal amplitude in one or more of the EEG bands using an EEG is called EEG biofeedback or neurofeedback. Minimum EEG voltages of 20-30 μV are seen in children and adults (Kraus et al., 2011).
Brainwaves Reflect Behavior
The ratio of slow (theta) to faster (beta) brainwaves shows how alert you are. This is the theta/beta ratio.
In the next section, we will examine delta, theta, rhythmic slow-wave, alpha, mu, synchronous "alpha," SMR, beta, high or fast beta, and gamma activity.
Local Versus Global Decision-making
The short time windows of fast oscillators facilitate local integration and decision-making, primarily because of the limitations of the axon conduction delays. The long time windows of slow oscillators can involve many neurons in large or distant brain areas and favor complex, global decisions.Delta (0.5-3 Hz)
There are two delta rhythms, a slow oscillation under 1 Hz and a traditional 1-4 Hz oscillation. The slow 0.3-0.4 Hz oscillation originates in the neocortex and persists when the thalamus is removed. Thalamo-cortical neurons generate the 1-4 Hz oscillations observed during human stage-3 sleep. Slow neocortical oscillations may synchronize the thalamic delta rhythm (Steriade, 2005).Delta activity is generated by cortical neurons when other connections do not activate them and is found predominantly in frontal areas. Delta is associated with sleep and infancy. Delta is associated with sleep and infancy. During stage-3 sleep, delta allows astrocytes to rebuild their stores of glycogen. Clinicians observe delta in clients diagnosed with ADHD, brain tumors, learning disorders, and traumatic brain injury (TBI). Rhythmic high-amplitude delta is associated with TBI, mainly if localized. Diffuse delta may be found in ADHD and learning disorders.
Normal Amplitudes
Delta should not be present in significant amounts in the awake adult EEG. "Apparent" delta is usually an eye movement artifact. Some delta activity probably occurs in the waking adult EEG.Delta bands are inhibited or down-trained but rarely rewarded. Delta desynchronization can be rewarded. The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 1-4 Hz activity from a 10-year-old male © John S. Anderson.
Theta (3-8 Hz)
The mechanisms that generate the theta rhythm are poorly understood. Theta differs depending on location and source. Amzica and Lopes da Silva (2011) consider the classic septal/diagonal band pacemaker model incomplete. Hippocampal interneurons, which innervate the hypothetical medial septum pacemaker, exercise top-down control. The hypothalamic supramammillary nucleus, with extensive connections to the brainstem, diencephalon, and medial septum, may also pace and modulate hippocampal theta. Further, a non-cholinergic theta source has been found within the entorhinal cortex of the hippocampus.Theta is associated with creativity, global synchronization, memory formation, and recall. Increased theta amplitudes correspond with hypo-perfusion and decreased glucose metabolism. Excessive frontal theta is linked with depression, daydreaming, distractibility, and inattention. A theta/beta (T/B) ratio of 3.0 may indicate ADHD depending on age, as T/B ratios are developmentally mediated (Monastra et al., 1999).
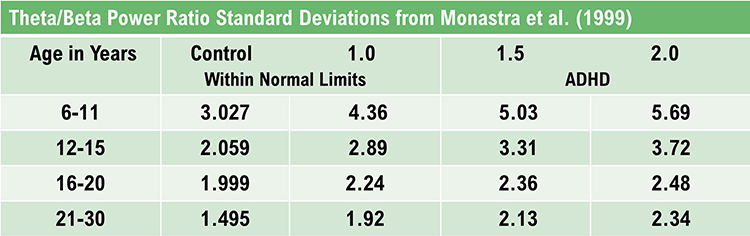
Normal Amplitudes
Theta voltage is age-related in the awake EEG. Voltage diminishes from age 8-30 with minimal amounts over age 30. A typical 6-7 Hz rhythm in the frontal midline (FCz) is associated with mental activity such as problem-solving and a wide variety of other functions. This rhythm appears to be limbic in origin. It is higher in amplitude and more synchronous when processing the feedback that an error has occurred. The 4-Hz rhythm is associated with childhood pleasurable experiences and memory searches in adults.Rhythmic Slow Wave (RSW or Theta)
Inhibit theta to remediate symptoms. Reward posterior RSW in alpha/theta training for addictions, global synchronization, optimal performance, and PTSD. RSW is generally not increased frontally. Clinicians may train for increases or decreases in phase synchrony. RSW is mainly seen in the frontal-midline (FCz) when awake with eyes open. The limbic system and thalamus generate RSW. Depending on location, RSW may be slowed-alpha as thalamic output slows.The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 4-8 Hz activity from a 10-year-old boy © John S. Anderson.
Alpha (8-13 Hz)
The 8-13-Hz alpha rhythm differs from spindle waves in both its source and the activity during which it is observed. Alpha 1 (low alpha) ranges from 8-10 Hz and alpha 2 (high alpha) from 10-13 Hz (Thompson & Thompson, 2016). Alpha rhythms depend on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons (Hughes & Crunelli, 2005). The alpha rhythm is maintained and propagated by cortical networks (Amzica & Lopes da Silva, 2018). Graphic of thalamocortical architecture courtesy of the Laboratory of Neuro Imaging and Martinos Center for Biomedical Imaging, Consortium of the Human Connectome Project.
Researchers have correlated the alpha rhythm with relaxed wakefulness. There are age- and function-related differences. Spindle waves, in contrast, originate in the thalamus and occur during unconsciousness and stage 2 sleep (Steriade, 2005).
Alpha is the dominant rhythm in adults and is located posteriorly. The 8-10 Hz range is associated with ADHD, daydreaming, fogginess, OCD, and TBI. Frontal asymmetry is associated with depression. The 10-12 Hz range is seen with inner calm (calm and alert) and meditation. Clinicians train alpha amplitude and phase synchrony up or down for remediation of symptoms, depending on location.
Posterior Dominant Rhythm (PDR)
The posterior alpha rhythm is visible at about 4 months with a frequency of around 4 Hz. Between 3-5 years, this rhythm is approximately 8 Hz with amplitudes as high as 100 μV. From 6-15 years, this rhythm is 9 Hz by age 7 and 10 Hz by ages 10-15 with a mean amplitude of 50-60 μV. Girls show a statistically faster acceleration of posterior alpha frequency than boys. From 13-21 years, the mean alpha frequency is 10 Hz, and amplitudes decline throughout this period. Faster alpha frequencies are associated with higher IQ and better memory performance.The following 19-channel BioTrace+ /NeXus-32 display of the response of the posterior dominant rhythm to eyes opening and closing © John S. Anderson.
Normal Amplitudes
The typical adult alpha frequency ranges from 9.5-10.5 Hz. Alpha below 8 Hz is considered abnormal. There are age-related differences. Alpha frequency declines after age 70. Adult amplitudes are 50 μV or less:60% have ~ 20-60 μV
28% have < 25 μV
6% have > 60 μV
Higher alpha amplitudes are observed over the non-dominant (right) hemisphere (alpha asymmetry). Most studies show no effect of handedness. Asymmetry is generally no more than 20 μV or 20% of the greater of the two amplitudes (Amzica & Lopes da Silva, 2018).
Causes of Excessive Alpha Amplitudes
Sleep deprivation or metabolic exhaustion can result in high amplitude and slowing of the peak frequency and persistent alpha during an eyes-open condition. Meditation practices can cause increased amplitudes and slowing, a faster alpha response to an eyes-closed condition, and persistent alpha in an eyes-open condition. Marijuana use and abuse can cause increased amplitudes and slowing, persistent alpha in an eyes-open condition, depending on the type of marijuana. These effects can persist for many years following abstinence.The following 19-channel BioTrace+ /NeXus-32 display of eyes-closed 8-12 Hz activity from a 13-year-old girl © John S. Anderson.
Mu Rhythm (7-11 Hz)
While the 7-11-Hz mu rhythm usually overlaps with the alpha range, its morphology deviates from the alpha waveform as one end is pointed. The mu rhythm can be recorded at C3 and C4 in a minority of subjects and may represent suppression of hand movement or imagining hand movement (Thompson & Thompson, 2016). Mu rhythms appear to regulate motor cortex activities via prefrontal cortical mirror neurons. These circuits may play a critical role in imitation learning and our ability to understand the actions of others. Mu rhythms facilitate the conversion of visual and auditory input into integrated skill-building functions. Attenuation of the mu rhythm appears to be associated with the activation of this function (Pineda, n.d.). The mu rhythm is highlighted below.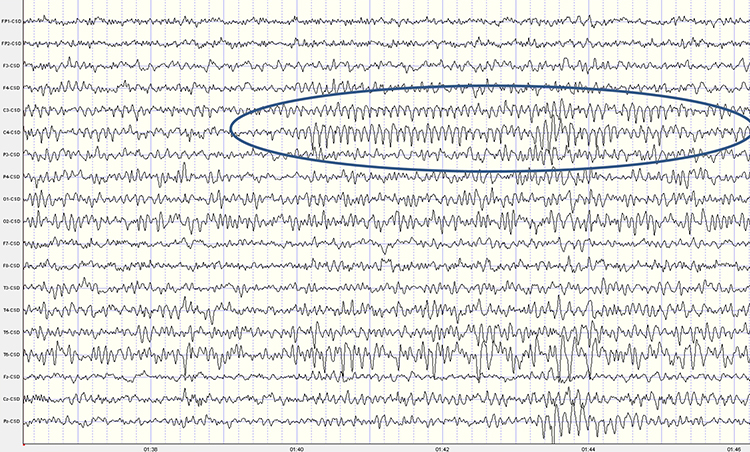
This second example of the mu rhythm shows a classic 10-11 Hz and 19-20 Hz "Owl Eye" presentation.
Synchronous "Alpha"
Various sensory systems such as our auditory, somatosensory, and visual systems produce localized and semi-independent "alpha" activity. Synchronous, distributed alpha integrates perception and facilitates action. Synchronous "alpha" appears to block the localized alpha-like patterns such as mu and the posterior rhythm in favor of more broadly distributed network integration.Sensorimotor Rhythm (13-15 Hz)
The sensorimotor rhythm (SMR) is beta 1 located on the sensorimotor strip (C3, Cz, C4). SMR amplitude increases when the motor circuitry is idle. SMR increases with stillness and decreases with movement. Deficient SMR may be observed in movement spectrum complaints like hyperactivity and tics. SMR appears as sleep spindles during stage-2 sleep. SMR is associated with neutral blood perfusion of the brain and resting levels of glucose metabolism. Clinicians typically reward increased SMR amplitude to calm hyperactivity and during theta/beta ratio training.The following 19-channel BioTrace+ /NeXus-32 display of 12-15 Hz activity © John S. Anderson.
Beta (over 12Hz)
Beta consists of rhythmic activity between 13-38 Hz. There are four beta ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz). Beta is located mainly in the frontal lobes. Beta is associated with focus, analysis, and relaxed thinking (Thompson & Thompson, 2015).Excessive beta is observed in anxiety, depression (asymmetry), insomnia, OCD, and sleep disorders. Deficient beta is seen in ADHD, cognitive decline, and learning disorders.
Since beta overlaps with the EMG range, clinicians must be careful when up-training this rhythm and use an EMG inhibit. Beta is generated by the brainstem and cortex and is associated with hyper-perfusion and increased glucose metabolism.
Normal 16-20+ Hz Beta Amplitudes
Beta amplitudes are minimal in children up to 12 years. There is a significant increase in beta amplitude and organization between 12-30 years. Beta is commonly seen in nearly all adults with amplitudes of 20 μV or less. Interhemispheric amplitude asymmetries exceeding 35% are abnormal. The following 19-channel BioTrace+ /NeXus-32 display of 13-21 Hz activity © John S. Anderson.Fast or High Beta Rhythms (20-35 Hz)
Fast 20-35-Hz oscillations are generated by activation of the mesencephalic reticular formation. Thalamocortical, rostral thalamic intralaminar, and cortical neurons spontaneously oscillate in this range. This activity is primarily seen in the frontal lobes and is associated with hyper-perfusion and increased glucose metabolism. Persistent excessive activity can lead to metabolic exhaustion.This activity may be associated with peak performance and cognitive processing and related to specificity and precision in information processing. Excessive high beta is associated with alcoholism, anxiety, OCD, rumination, and worry. Clinicians often inhibit high beta activity but rarely reward it.
The following 19-channel BioTrace+ /NeXus-32 display of eyes-closed ~ 25 Hz fast beta activity © John S. Anderson.
Gamma Rhythms (28-80 Hz)
Amzica and Lopes da Silva (2011) concluded that gamma oscillations might speed information distribution and processing. Gamma bursts occur during problem-solving, and the absence of gamma is associated with cognitive deficits and learning disorders. Gamma synchrony is related to cognitive processing and is essential in coding by contributing specificity and precision to information processing. Gamma is theorized to serve as a "binding rhythm" that integrates sensory inputs into perception and consciousness.The following 19-channel BioTrace+ /NeXus-32 display of eyes-open 36-44 Hz activity in a 10-year-old boy © John S. Anderson.
Gamma rhythms are linked with SCPs. The following BioTrace+ /NeXus-32 display of SCP and gamma activity © John S. Anderson.
GENERAL CORTICAL AND SUBCORTICAL ANATOMY
Major Divisions
The human nervous consists of the central nervous system and peripheral nervous system. The central nervous system (CNS) consists of the brain, spinal cord, and retina.
The 3-pound brain consists of approximately 86 billion neurons (Chan et al., 2009; Voytek, 2013). Graphic © Jasada Sabai/Shutterstock.com.
The cylindrical spinal cord consists of nervous tissue that extends from the medulla (brainstem) to the lumbar (lower back) segment of the vertebral column. The spinal cord distributes sensory information from the body to the brain, and CNS commands from the brain to the body. The spinal cord also contains networks that control reflexes and central pattern generators. Graphic © Sabastian Kalulitzki/ Shutterstock.com.
The peripheral nervous system (PNS) consists of neurons and nerves outside of the brain and spinal cord. The peripheral nervous system is comprised of the autonomic nervous system and somatic nervous system. The graphic below is from the Connexions website.
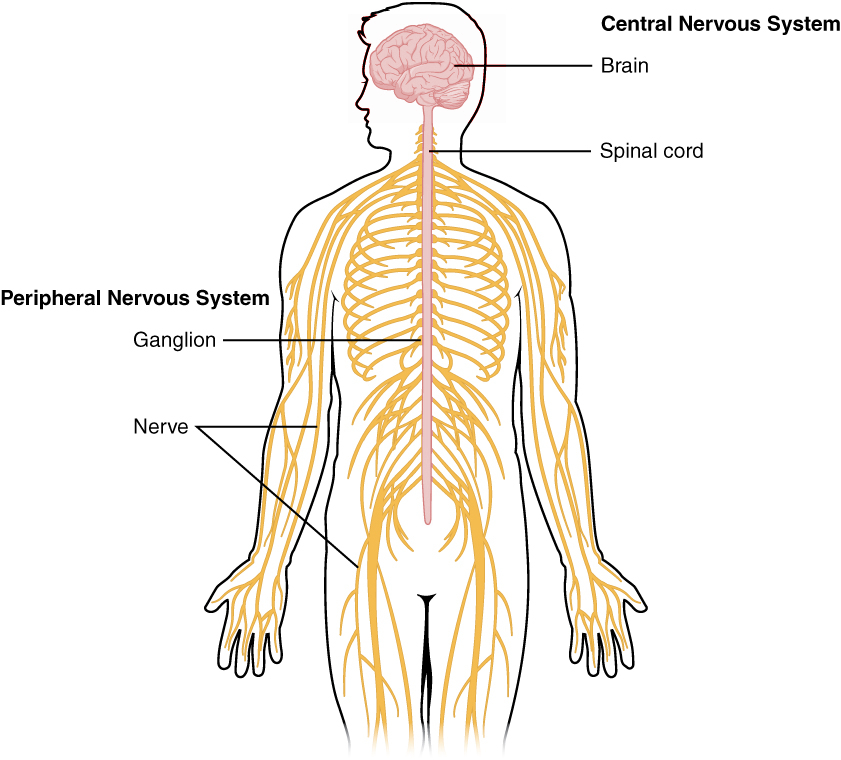
Nerves
Nerves are bundles of axons that lie outside of the central nervous system. Motor nerves distribute instructions from the CNS to the rest of the body. Sensory nerves transmit information from sensory receptors to the CNS.There are three major systems of nerves: cranial nerves, spinal nerves, and the autonomic nervous system. The 12 pairs of cranial nerves distribute sensory and motor information. There are three exclusively sensory pathways to the brain: olfactory (I), optic (II), and vestibulocochlear (VIII). There are five exclusively motor pathways from the brain: oculomotor (III), trochlear (IV), abducens (VI), spinal accessory (XI), and hypoglossal (XII). Finally, four cranial nerves carry sensory and motor information: trigeminal (V), facial (VII), glossopharyngeal (IX), and vagus (X). Check out the Blausen Cranial Nerves animation. Graphic © Alila Medical Media/Shutterstock.com.
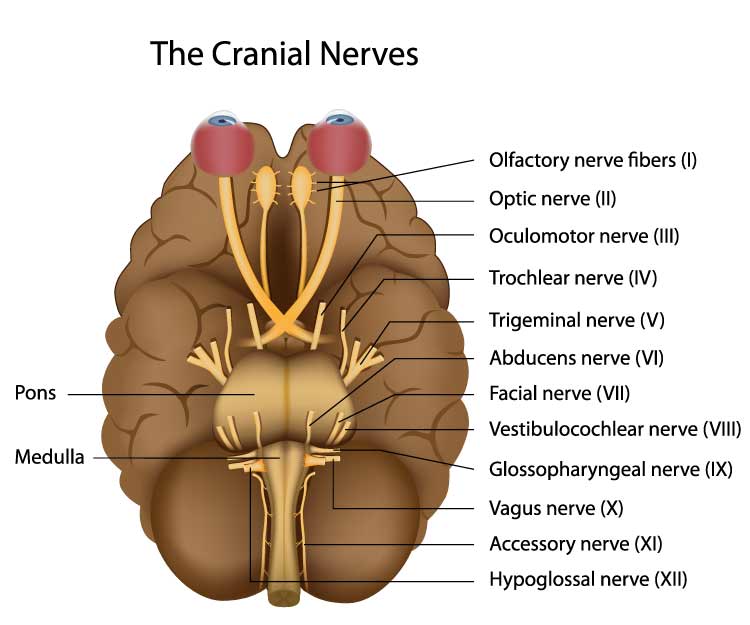
Thirty-one pairs of spinal nerves, each member serving one side of the body, leave the spinal cord through openings in the backbone. Check out the Blausen Spinal Nerves and Spinal Cord: Sensory and Motor Signals animations. Graphic © yodiyim/Shutterstock.com.
Each spinal nerve carries sensory projections from the body (dorsal root) and motor commands from the spinal cord to skeletal muscles (ventral root). Graphic © Alila Medical Media/Shutterstock.com.
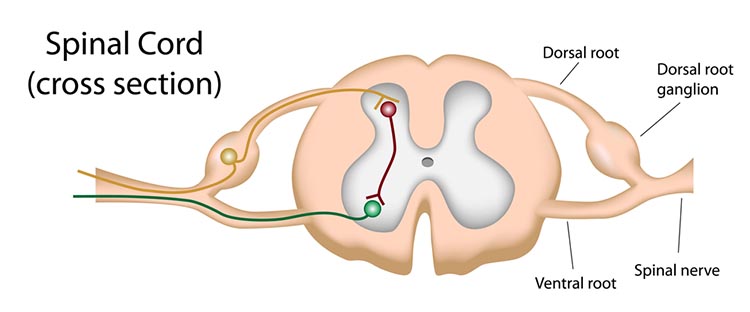
Autonomic Nervous System
The autonomic nervous system regulates cardiac and smooth muscle and glands, transmits sensory information to the CNS, and innervates muscle spindles. The autonomic nervous system is the brain’s main system for monitoring and controlling major organs. Check out the Blausen Autonomic Nervous System animation.While we normally do not exercise very much intentional, conscious autonomic control, self-regulation disciplines like yoga and animal and human research in biofeedback have shown that we can teach voluntary autonomic control of heart rate variability, blood flow to our fingers, toes, and scalp, and finger sweat gland activity to treat disorders and achieve optimal performance.
The autonomic nervous system is divided into three main systems: sympathetic, parasympathetic, and enteric. Check out the YouTube video, The Autonomic Nervous System.
We activate the sympathetic nervous system when we encounter a threat that we can fight or flee. However, we also activate this branch when we get up from our couch and when we exercise. Check out the Blausen Sympathetic Nervous System animation.
The parasympathetic system can oppose or complement sympathetic activity. When we feel safe, the parasympathetic branch allows us to self-regulate (meditate or use neurofeedback skills), socially engage with others, and engage in executive functions like planning. When we feel endangered and cannot fight or flee, this system produces freezing (think mouse in the jaws of a cat), fainting responses, or dissociation responses. Check out the Blausen Parasympathetic Nervous System animation.
The enteric system consists of over 100 million neurons that release over 30 neurotransmitters to control the gut under CNS control. This system helps to maintain fluid and nutrient balance.
Somatic Nervous System
The somatic nervous system is comprised of spinal nerves that innervate somatosensory receptors in the skin, joints, and skeletal muscles. While somatic motoneuron cell bodies lie in the CNS, most of their axons are in the PNS. The cell bodies of somatic sensory neurons are in the PNS dorsal root ganglia. Dorsal root graphic © stihii/Shutterstock.com.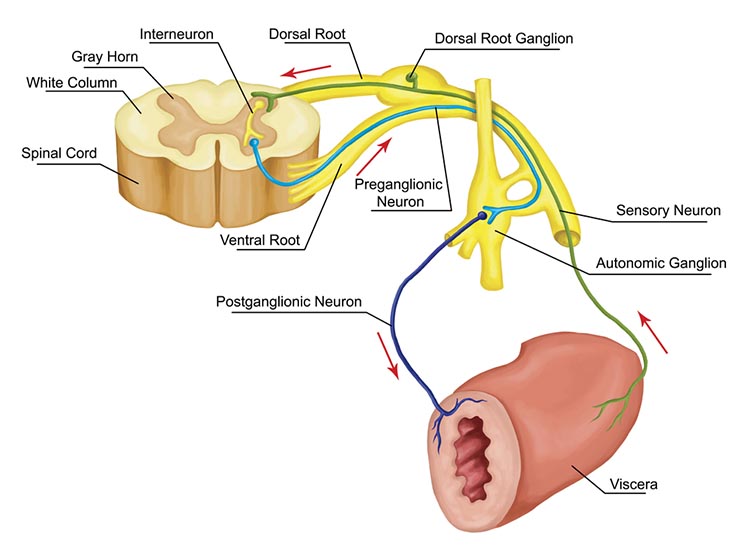
Navigating the Brain
Orientations
Three customary planes for viewing the body and brain are sagittal, coronal, and horizontal. The sagittal plane divides the body into right and left halves. The coronal plane separates the body into front and back parts. Finally, the horizontal (transverse) plane divides the brain into upper and lower parts (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.
Directional Terms
Important directional terms include medial (toward the middle) and lateral (toward the side), ipsilateral (same side) and contralateral (opposite side), superior (above) and inferior (below), anterior/rostral (toward the head), and caudal (toward the tail), proximal (near the center) and distal (toward the periphery, and dorsal (toward or at the back) and ventral (toward the belly) (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.
Cortical Features
The adult human brain has a volume of about 1100 cm2 and requires convolutions to fit within the skull (Bear, Connors, & Paradiso, 2020). Two-thirds of the cortical surface lies within these folds (Breedlove & Watson, 2020). Anatomists distinguish three topographical features of the cerebral cortex: gyrus, sulcus, and fissure. Check out the Blausen Cerebral Landmarks animation.A gyrus is a ridged area of the brain. The precentral gyrus, which is anterior to the central sulcus, is the primary motor cortex (controls muscles and movements). The postcentral gyrus, which is posterior to the central sulcus, is the primary somatosensory cortex (receives somatosensory information).
A sulcus is a groove in the cortical surface. As we just observed, the central sulcus separates the primary motor cortex from the primary somatosensory cortex. A fissure is a deep groove. The Sylvian fissure (also called the lateral fissure or lateral sulcus) is the upper boundary of the temporal lobe (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.
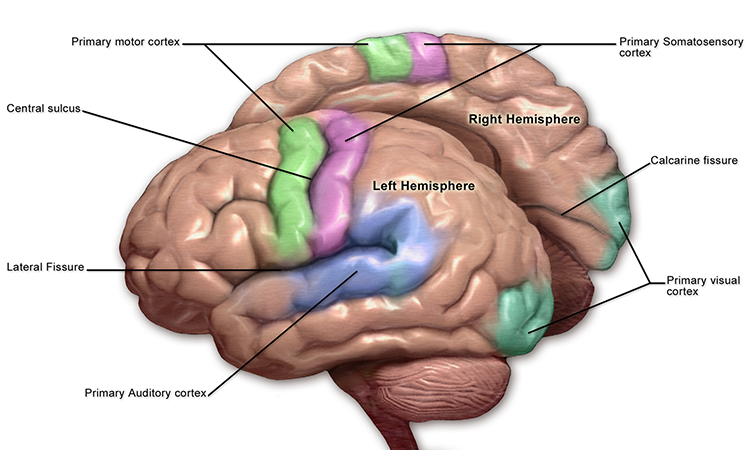
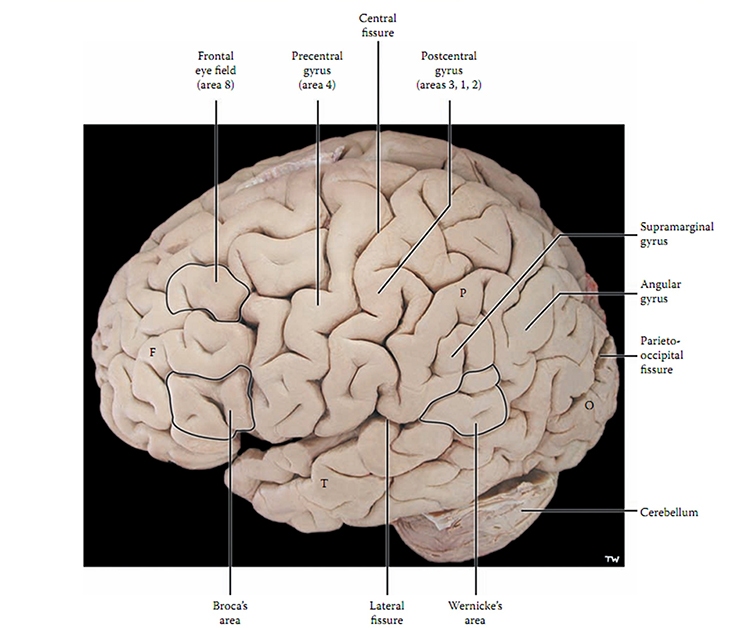
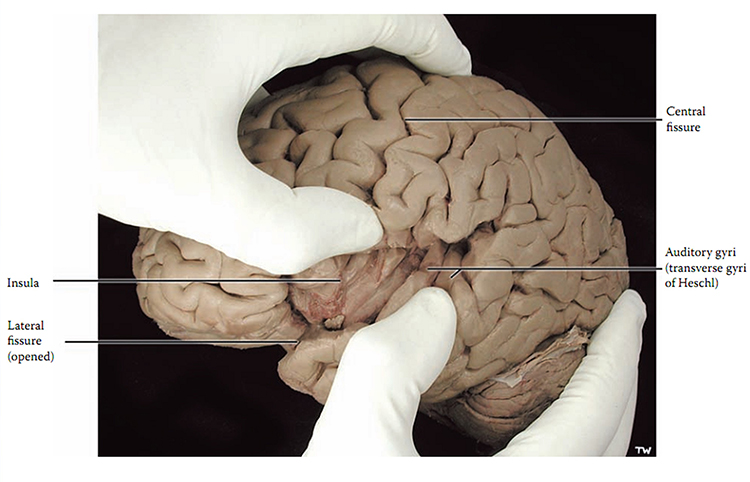
The Unfixed Brain
This video was produced by Suzanne Stensaas, PhD, Department of Neurobiology and Anatomy, and the Spencer S. Eccles Health Sciences Library, University of Utah.Dissecting Brains
This video is courtesy of the Wellcome Collection.Subdivisions of the Brain
The brain is divided into three major subdivisions: forebrain, midbrain, and hindbrain. Graphic © 2020 Breedlove and Watson/Sinauer.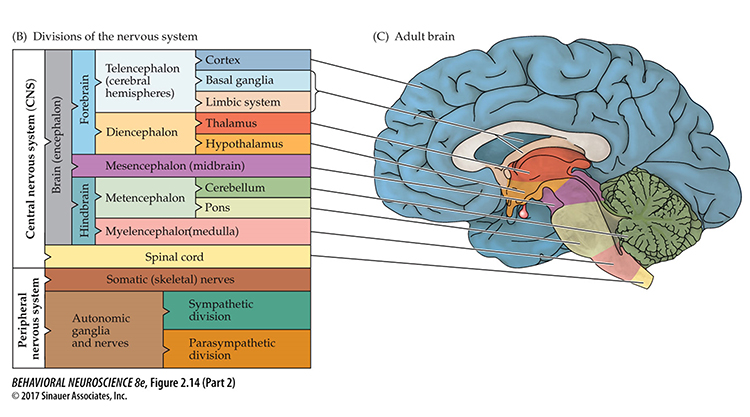
The forebrain consists of the telencephalon (cerebral hemispheres) and the diencephalon. The telencephalon encompasses the cerebral cortex and the deeper structures of the basal ganglia and limbic system. Check out the Blausen Limbic System animation. Limbic system graphic © SciePro/Shutterstock.com.
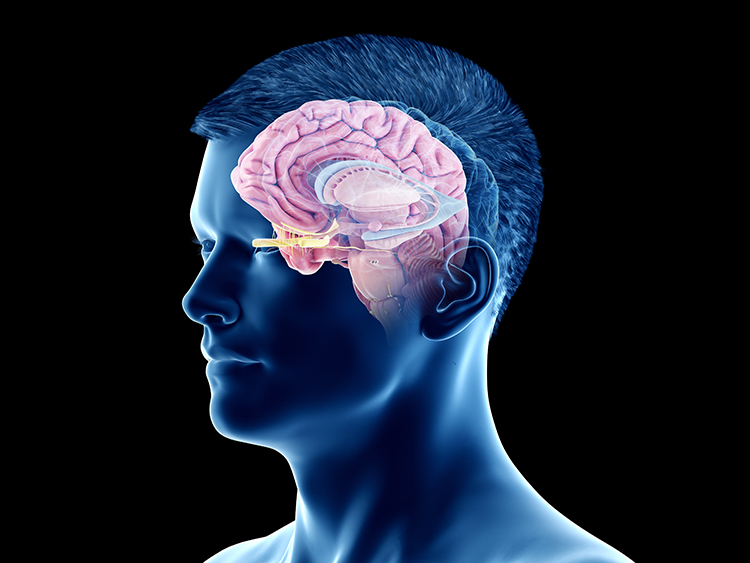
The diencephalon in the posterior forebrain contains the thalamus and hypothalamus. Check out the Blausen Hypothalamus animation. Thalamus graphic © SciePro/Shutterstock.com.
The midbrain consists of the mesencephalon, which includes the inferior colliculi, superior colliculi, and substantia nigra. The degeneration of the substantia nigra is a key step in developing Parkinson's disease. Substantia nigra graphic © Kateryna Kon/Shutterstock.com.
The hindbrain contains the metencephalon and myelencephalon. The metencephalon is comprised of the cerebellum and pons. The cerebellum plays a role in higher-level functions like emotional and cognitive regulation, speed, capacity, consistency, and appropriateness of cognitive and emotional processes. Damage to the cerebellum can reduce general intelligence. Check out the Blausen Cerebrum and Cerebellum animation. Cerebellum graphic with highlighted Purkinje neuron © Kateryna Kon/Shutterstock.com.
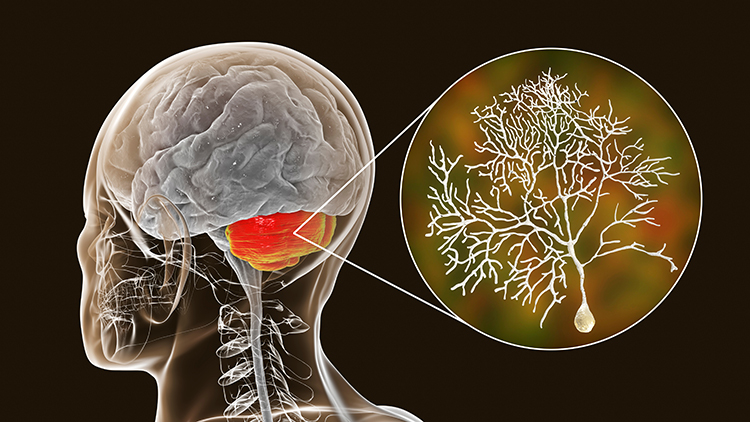
The myelencephalon consists of the medulla. The medulla plays a critical role in the speeding and slowing of the heart across each breathing cycle, a phenomenon called respiratory sinus arrhythmia or RSA. Alcohol, opioids, and sedative-hypnotics can fatally depress brainstem respiratory centers slowing and halting breathing. Check out the Blausen Brainstem animation. Medulla graphic © mkfilm/Shutterstock.com.
Meninges
Three meninges protect the brain and spinal cord, which are housed within the skull and vertebrae. These membranes include the dura mater, pia mater, and arachnoid (Breedlove & Watson, 2020). Graphic © Alilia Medical Media/Shutterstock.com.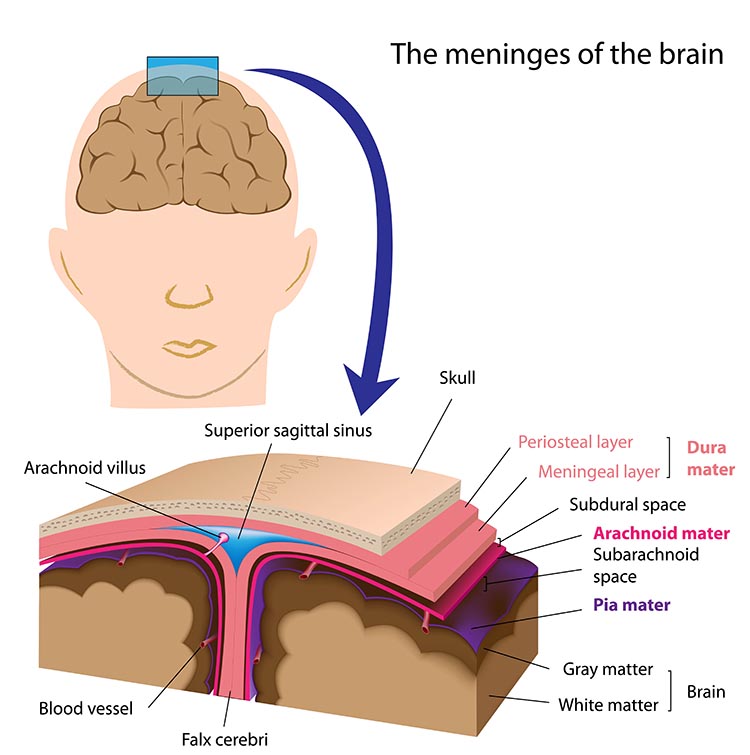
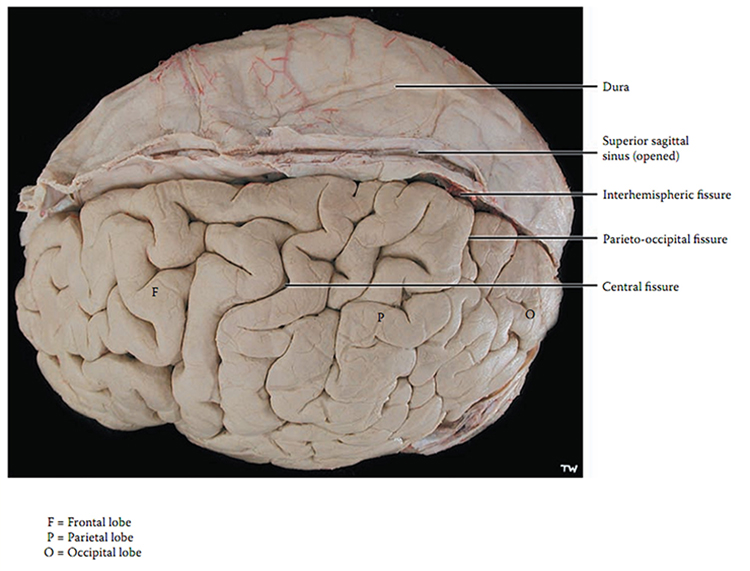
Cerebral Ventricles
The cerebral ventricles are a network of fluid-filled chambers that protect the brain from trauma due to abrupt head movements and that facilitate the exchange of nutrients and wastes between blood vessels and the brain. These cavities, found within all four lobes of each hemisphere, include the lateral, third, and fourth ventricles. The ventricular system circulates cerebrospinal fluid (CSF) produced by the choroid plexus membrane of the lateral ventricles (Breedlove & Watson, 2020). Check out the Blausen Ventricles of the Brain animation. Graphic © joshya/ Shutterstock.com.
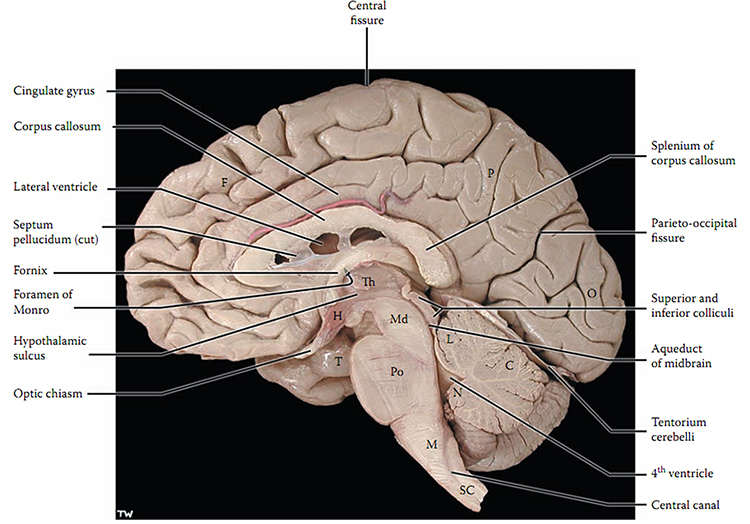
Glymphatic System
The glymphatic system is a newly discovered lymphatic system in the brain. This recently discovered system provides a flow of CSF through the brain's interior that helps clear cellular debris, proteins, and other wastes. In the diagram below: (1) cerebrospinal fluid (CSF) flows from the subarachnoid space to travel outside pulsing arteries, (2) CSF enters the brain via aquaporins and collects waste, and (3) CSF enters the perivascular space surrounding capillaries and is removed by venous circulation.
By removing harmful substances such as the amyloid proteins implicated in Alzheimer’s disease, the glymphatic flow may protect us from various neurological disorders (Breedlove & Watson, 2020). The glymphatic system removes most of its waste during stage 3 sleep, called slow-wave sleep.
The Brain's Vascular System
The resting brain consumes over 20% of the body's energy. TThe internal carotid artery's anterior and middle cerebral arterial branches deliver blood to about two-thirds of the cerebral hemispheres. The posterior cerebral arteries' left and right posterior vertebral arterial branches supply blood to the posterior cerebral hemispheres, cerebellum, and brainstem (Breedlove & Watson, 2020). Graphic © Alilia Medical Media/Shutterstock.com.
The effects of a stroke due to blood vessel blockage or rupture are limited because paired arteries supply each brain hemisphere. The circle of Willis, located at the base of the brain, is a vascular network comprised of the carotid and basilar arteries. This structure may provide another route for delivering blood when a major artery is compromised by disease or traumatic injury. Graphic © Alilia Medical Media/Shutterstock.com.
MAJOR FUNCTIONS OF CORTICAL LOBES AND MAJOR SUBCORTICAL STRUCTURES AND BRODMANN AREAS
Cortical Lobes
The cortical lobes are named for the overlying bones of the skull (Breedlove & Watson, 2020). Graphic © Sebastian Kaulitzki/Shutterstock.com.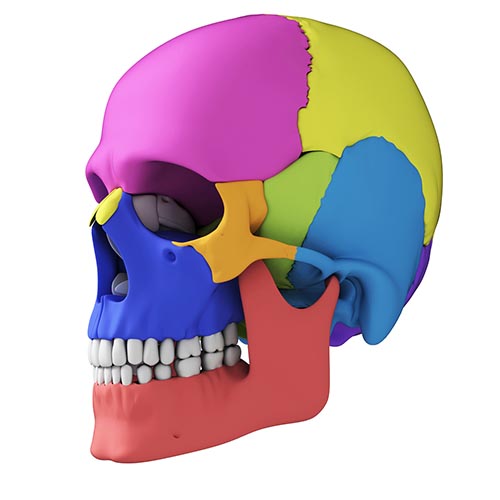
The cortex is required for executive functions like attention, planning, and problem-solving.
Without a cerebral cortex, a person would be blind, deaf, dumb, and unable to initiate voluntary movement (Bear, Connors, & Paradiso, 2015, p. 205).
The five major cortical regions include the frontal, parietal, temporal, and occipital lobes, and the insula (not shown). Cortical lobes graphic © Madrock24/Shutterstock.com.
Frontal Lobes
The frontal lobes (F7, F3, Fz, F8, F4) consist of the cortex anterior to the central sulcus and consist of the primary motor cortex, motor association cortex, Broca's area, and prefrontal cortex. Check out the Blausen Frontal Lobe and Frontal Pole animations.Essential left frontal lobe functions include working memory, concentration, planning, and positive emotion. The main clinical concern is Major Depressive Disorder (MDD).
Critical right frontal lobe functions include declarative memory, social awareness, and negative emotions. The main clinical concerns include Generalized Anxiety Disorder (GAD), fear, and impaired executive functioning.
Frontal lobe damage may result in impaired flexibility and problem solving, increased risk-taking, changes in social behavior, an inability to use external cues, and deficits in emotional self-regulation. Graphic © decade3d - anatomy online/Shutterstock.com.
.jpg)
The primary motor cortex is located in the precentral gyrus (Brodmann area 4, BA 4). It organizes the opposite side of the body's muscles and movements required for the fine motor coordination required by tasks like writing. Lesions can result in loss of motor control, including rigid paralysis. Graphic © 2020 Breedlove and Watson/Sinauer.
The motor association cortex (premotor cortex) is rostral to the primary motor cortex (BA 6) and helps program and execute movements. The motor association cortex is the piano player, and the primary motor cortex is the piano keyboard (Carlson & Birkett, 2016). The primary and motor association cortex collectively appear to map behaviors instead of specific muscles or movements (Breedlove & Watson, 2020).
Broca's area, which is located in the inferior frontal gyrus (BA 44 and 45) of the dominant hemisphere (F7-T3 in the left hemisphere), is concerned with speech production, grammar, language comprehension, and sequencing (Caplan, 2006). Lesions to Broca's area can produce dyslexia, deficits in grammar, spelling, and reading, and Broca's aphasia. Broca's area receives input from Wernicke's area via the arcuate fasciculus (Breedlove & Watson, 2020). Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.
The prefrontal cortex (PFC) is rostral to the motor association area (BA 9, 10, 11, 12, 46, 47). It is responsible for executive functions, which involve attention, working memory, prediction of the outcomes of current and hypothetical actions, working toward goals, problem-solving, planning, and the ability to suppress actions that could lead to unwanted outcomes (Diamond, 2013). The PFC integrates emotion and reward in decision-making (Fuster, 2015).
Important subdivisions of the PFC include the orbitofrontal cortex, ventromedial, and dorsolateral PFC.
The orbitofrontal cortex (OFC) consists of Brodmann area (BA 10, 11, and 47) (Kringelbach, 2005). The OFC may aid planning by evaluating the consequences (rewards and punishments) of our actions and helping to generate the motivation to ingest drugs. Phineas Gage's profound personality changes were produced by damage to this subdivision and the ventromedial PFC (VMPFC). The OFC appears to adjust decision-making based on the stakes involved and enables us to switch between significant (investments) and trivial (snacks) choices. Finally, the OFC compares our current options with recent ones, while the anterior cingulate cortex registers our predictions and prediction errors (Kennerley et al., 2011).
The ventromedial prefrontal cortex (VMPFC) corresponds to the ventromedial reward network (Ongur & Price, 2000) and includes BA 10, 14, 25, 32, and parts of 11, 12, and 13. The VMPFC is implicated in making decisions, where the outcomes are uncertain and moral values must be applied to actual situations. Patients with damage to the VMPFC choose outputs that lead to immediate reward, regardless of their future cost. They do not learn from their mistakes. Since they have difficulty understanding social cues, they may not recognize deception, irony, or sarcasm (Zaid & Andreotti, 2010). Likewise, they may not control their emotional reactions in social situations, particularly anger and violence (Carlson & Birkett, 2016).
The dorsolateral prefrontal cortex (DLPFC) is located in the middle frontal gyrus and includes BA 9 and 46. The DLPFC shares responsibility with cortical and subcortical networks for executive functions like abstract reasoning, cognitive flexibility, decision-making, inhibition, planning, and working memory (Miller & Cummings, 2007). It exercises the highest cortical level of motor control (Hale & Fiorello, 2004).
The left DLPFC is concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals. The right DLPFC organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location. In unipolar depression and premenstrual dysphoric disorder, the right DLPFC may be more active than the left (alpha asymmetry).
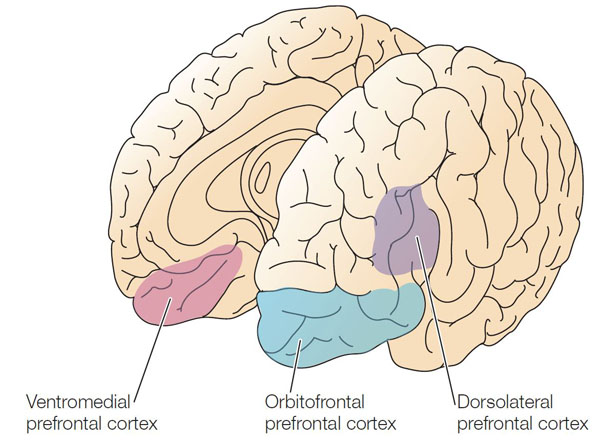
Anterior Cingulate Cortex (ACC)
The cingulate cortex has reciprocal connections with the parahippocampal gyri, integrates limbic functions, and is part of the salience network. Cingulate cortical functions include child nurturing, grooming, play, and organization and managing input/output functions. Check out the Blausen Cingulate Gyrus animation.The anterior cingulate cortex (ACC) (Fpz, Fz, Cz, Pz) lies above the corpus callosum (BA 24, 32, 33). The dorsal ACC is connected to both the PFC and parietal cortex. The ACC plays a vital role in attention and is activated during working memory. The ACC mediates emotional and physical pain, and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components. Graphic courtesy of Geoff B. Hall in Wikimedia Commons.
The Stroop test illustrates a cognitive monitoring task where color and names conflict. Discrepancies between facial and vocal cues show an affective conflict. The anterior cingulate recruits other brain areas to resolve these conflicts.
The anterior cingulate gyrus helps us allocate attention to focusing on a target and then disengaging, perceiving options, and making adaptive choices. The anterior cingulate gyrus, the prefrontal cortex, and the caudate function abnormally in children diagnosed with ADHD during selective attention tasks. fMRI evaluation showed that neurofeedback could teach children to normalize activity in these structures (Beauregard & Levesque, 2006).
The anterior cingulate gyrus is involved in motivation and the perception of emotional and physical pain. Eisenberger, Lieberman, and Williams (2003) used an fMRI to study the brains of subjects who believed that two companions playing an on-line baseball simulation suddenly dropped them from the game. Their emotional distress activated the anterior cingulate cortex, which evaluates the unpleasantness of physical pain. de Charms and colleagues (2005) provided real-time fMRI feedback from the anterior cingulate to subjects. They learned to reduce its metabolism and the intensity of experimental pain.
Lesions to the cingulate can produce akinetic mutism, in which a person cannot produce orienting responses. Cingulate malfunction can result in addictive behaviors (alcohol or drug abuse, eating disorders, chronic pain), obsessive-compulsive disorder and OCD spectrum disorders, and “road rage.”
Parahippocampal Gyri
The parahippocampal gyri are located within the medial temporal lobe. The parahippocampal gyri form spatial and nonspatial contextual associations, which serve as building blocks for contextual processing, episodic memory, navigation, and scene processing (Aminoff, Kveraga, & Bar, 2013). They may also play a role in emotional responsiveness.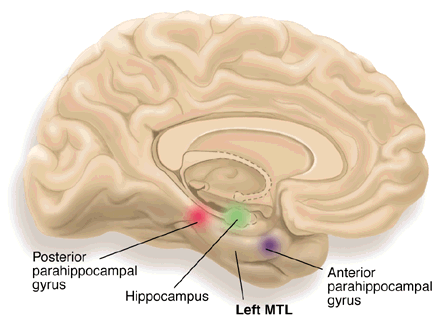
Parietal Lobes
The parietal lobes (Pz, P3, P4) are posterior to the frontal lobes (BA 1, 2, 3, 5, 7, 39, 40) and are divided into the primary somatosensory cortex and secondary somatosensory cortex. Their main function is to process somatosensory information like pain and touch.Major left parietal lobe functions include problem-solving, math, complex grammar, attention, and association. Essential right parietal lobe functions include spatial awareness and geometry. Graphic © decade3d - anatomy online/Shutterstock.com.
The primary somatosensory cortex (S1) is located in the parietal lobe's postcentral gyrus posterior to the central sulcus (BA 3, 1, and 2). S1 maps touch and pain information from the opposite side of the body. The secondary somatosensory cortex (S2) is adjacent to S1 (BA 40 and 43), receives projections from it, and maps touch and pain from both sides of the body (Breedlove & Watson, 2020). Graphic by Paskari from Wikimedia Commons.
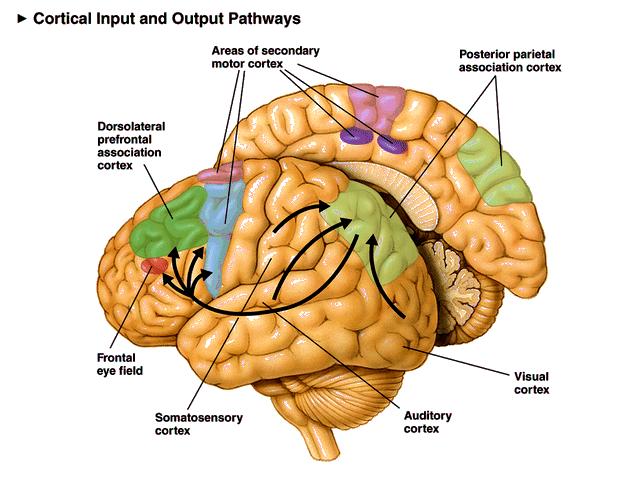
The primary function of the parietal lobes is to process somatosensory information like pain and touch. The parietal cortex monitors our preparation for a movement and is responsible for our subjective feeling of intending to move (Sirigu et al., 2004).
The angular gyrus, located near the superior temporal lobe (BA 39), is involved in reading, math, and copying writing. The graphic below highlights the angular gyrus in red © Kateryna Kon/Shutterstock.com.
Major left hemisphere functions include attention, association, complex grammar, math, object names, and somatosensation.
Major right hemisphere functions include body boundary, geometry, guiding reaching with the hands, somatosensation, and spatial perception (Demos, 2019).
Temporal Lobes
The temporal lobes (T3, T4, T5, T6) are separated from the rest of the cortical lobes by the Sylvian fissure (BA 15, 20, 21, 22, 37, 38, 39, 40, 52). The temporal lobes process hearing, smell, and taste information and help us understand spoken language and recognize visual objects and faces (Breedlove & Watson, 2020).Wernicke's area, located in the temporoparietal cortex (BA 22) of the dominant hemisphere, is specialized for speech perception and production. Damage can result in an inability to understand the meaning of speech and to construct intelligible sentences. Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.

Major left hemisphere functions include affect, declarative memories, language comprehension, perception of movement, reading, and word recognition.
Important right hemisphere functions include face and object recognition, music, and social cues (Demos, 2019).
Occipital Lobes
The occipital lobes (Oz, O1, O2) are posterior to the parietal lobes. The primary visual cortex (VI) is located within the calcarine sulcus (BA 17). The occipital lobes process visual information from the eyes in collaboration with the frontal, parietal, and temporal lobes. Graphic © decade3d - anatomy online/Shutterstock.com.
Their primary functions are visual and include the analysis of orientation, color, spatial frequency, illusory contours, and complex patterns like concentric and radial stimuli (Breedlove & Watson, 2020).
Insular Cortex
The insular cortex lies deep within the lateral sulcus that divides the temporal and parietal lobes (BA 13). The insula is involved in emotional and autonomic responses to external stimuli and is part of the salience network. The insula detects salient events via afferent pathways and switches between other large-scale networks when such events are identified, affecting attention and working memory. The anterior and poster insulae interact to regulate autonomic responses to salient stimuli. Interactive communication between the insula and anterior cingulate cortex facilitates motor control (Menon & Uddin, 2010). The right insula mediates awareness of our body, empathy, and understanding others’ points of view (Khazan, 2019). Check out the Blausen Cerebral Cortex: Insula animation.Increased heart rate variability strengthens the connectivity between the ACC and the insula for empathy and the ability to understand others’ emotions, feel gratitude, socially connect, understand our own psychophysiological states, and restore nervous system balance. Mindfulness meditation increases insula gray matter and activation.
The insula functions as an integrative and organizational hub for the salience network. The insula integrates interoceptive awareness, emotional experience, and external perception to facilitate our global perception of the world and relationship with it. The insula directs specific networks in the processing of salient stimuli and in generating appropriate behavioral responses to these stimuli (Wiebking & Northoff, 2014).
The insula is the primary taste cortex and is activated when you see something that disgusts you (a fraternity bathroom) or see another person’s expression of disgust. Pictures of lovers also activate the anterior insula as opposed to friends. When the anterior insula was activated in neuroeconomic studies, subjects chose risk-avoidant financial strategies (choosing bonds instead of stocks). In the Prisoner’s Dilemma game, mutually cooperative decisions also resulted in the activation of this region.
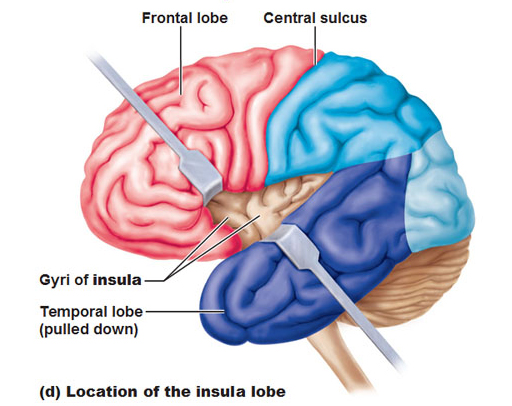
Antonio Damasio has proposed that this region helps map visceral states associated with emotional experience and generate conscious feelings. This could provide the basis of somatic markers like the discomfort produced by a risky decision.
The insular cortex has been implicated in the experience of pain and basic emotions, including anger, disgust, fear, happiness, and sadness. The insular cortex receives reports of internal states, like hunger and drug craving, and motivates individuals to engage in consummatory behavior. The insular cortex plays a crucial role in craving and impulse control. This region is stimulated by drug-related cues and may activate memories of pleasurable drug-related experiences. Stroke damage to the insular cortex (see red area below) can eliminate nicotine addiction. Graphic from National Institute of Drug Abuse found in Wikimedia Commons.
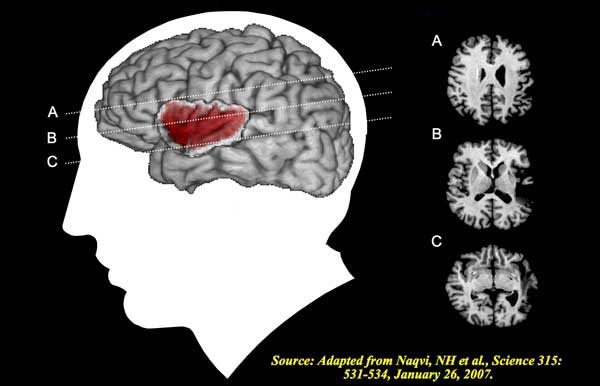
Mirror Neuron System
Researchers have proposed that the human neocortex contains a mirror neuron system that is selectively activated when we perform movements or observe the actions of others. In humans, this system may encompass Broca's area, the inferior frontal gyrus, inferior parietal cortex, insular cortex, occipital cortex, premotor cortex, and superior temporal sulcus.Molenberghs et al. (2011) unexpectedly found neurons with mirroring properties in the cerebellum, limbic system, and primary visual cortex. Graphic courtesy of Wikimedia Commons.
The authors proposed that a core network is responsible for observing and executing movements. The nervous system recruits additional areas to perform non-motor affective, auditory, and somatosensory functions.

Mirror neurons look like other neurons when examined using a microscope. Their mirror properties emerge from their sensory, motor, and emotional systems connections. Perhaps most mirror neurons may be tuned by experience (Catmur, Walsh, & Heyes, 2007).
The mirror neuron system appears to encode the goal of a motor act and its component movements, whether a model manipulates an object or mimes the action. The mirror neuron system encodes the actions of others and stores them to predict their future actions (Rajmohan & Mohandas, 2007).
Soon after birth, an immature mirror neuron system may allow babies to imitate their parents' mouth movements, like thrusting out the tongue. Graphic courtesy of Wikimedia Commons.

Ramachandran (2011) has called the mirror neurons activated when they observe others' movements "monkey see—monkey do neurons." He calls mirror neurons activated by others' emotional displays "Gandhi neurons." Check out Ramachandran's TED Talk, The Neurons that Shaped Civilization.

Rizzolatti and Sinigaglia (2008) hypothesized that the primary role of the mirror neuron system is to help us understand others’ intentions, which allows us to achieve empathy. When we observe others’ facial expressions of emotion, visual information may be directly transmitted to mirror neurons in the insula, producing the visceral changes that color our emotions. In autism, mirror neurons may not fire when observing other individuals performing actions. This may help explain deficits in empathy, social skills, language, and the development of a theory of mind (Enticott et al., 2011).
Cortical and Subcortical Connections
Neocortical zones are connected with cortical and subcortical regions by specialized fiber tracts: association bundles, projection fibers, and commissural bundles. A meta-networking model proposes that the dynamic interaction of "distributed but relatively specialized networks" mediates brain functions like language (Herbet & Duffau, 2020, p. 1181). Graphic © American Physiological Society.
Caption: High-level distributed networks mediate higher-order cognitive and emotional functions using local and network integration of specialist areas. Transient meta-networks, created by cross-network integration, are responsible for functional plasticity in behavior and cognitions, creating novel solutions to problems.
Association Bundles
Association bundles link cortical regions located in the same hemisphere using U-shaped white matter tracts. These include the arcuate fasciculus (AF), frontal aslant tract (FAT), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fascicle (ILF), middle longtitudinal fasciculus (MdLF), superior longitudinal fasciculus (SLF), and uncinate fasciculus (UF).
Tractography showing arcuate fasciculus courtesy of Wikipedia.Yeh, F. C., Panesar, S., Fernandes, D., Meola, A., Yoshino, M., Fernandez-Miranda, J. C., ... & Verstynen, T. (2018). Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage, 178, 57-68
Projection Fibers
Projection fibers connect the cortex with structures deep in the brain, the brainstem, and spinal cord. The frontostriatal tract (FST) connects the premotor cortex with the caudate nucleus and putamen, thalamocortical, optic, and pyramidal tracts.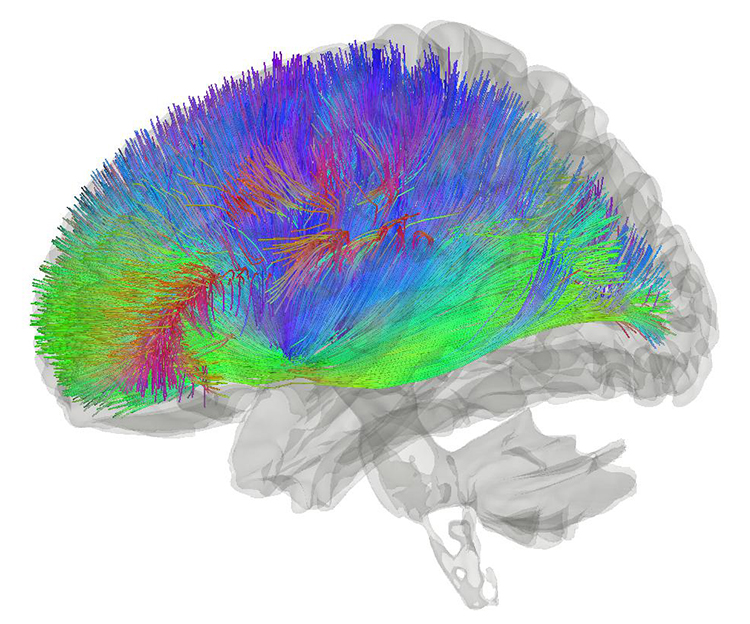
Caption: Tractography showing corticostriatal connections courtesy of Wikipedia. Yeh, F. C., Panesar, S., Fernandes, D., Meola, A., Yoshino, M., Fernandez-Miranda, J. C., ... & Verstynen, T. (2018). Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage, 178, 57-68
Commissural Bundles
The left and right hemispheres communicate using three commissures or axon tracts. The corpus callosum is the largest tract and connects the left and right frontal, parietal, and occipital lobes. Certain conditions, such as prenatal exposure to alcohol and other drugs, may result in agenesis of the corpus callosum, in which part or all of this fiber bundle is missing. Graphic © decade3d - anatomy online/Shutterstock.com.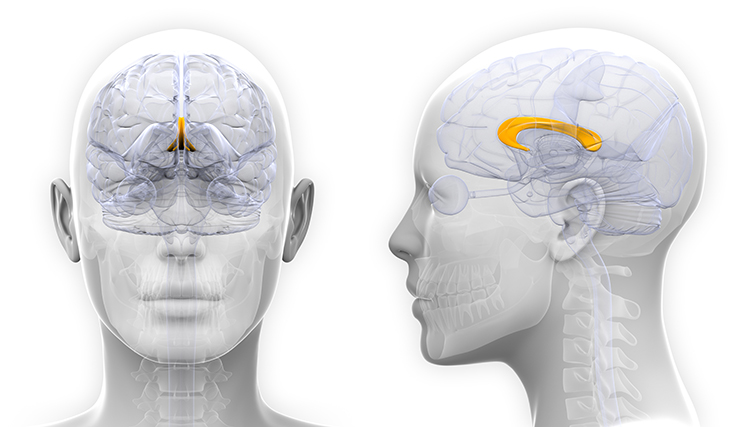
The anterior commissure, shown above the third ventricle at the bottom of the diagram, is considerably smaller than the corpus callosum and connects the left and right temporal lobes and the hippocampus and amygdala. The posterior commissure, located below the corpus callosum, connects the right and left diencephalon and mesencephalon (Breedlove & Watson, 2020). Graphic courtesy of Operative Neurosurgery Anterior Commissure article.
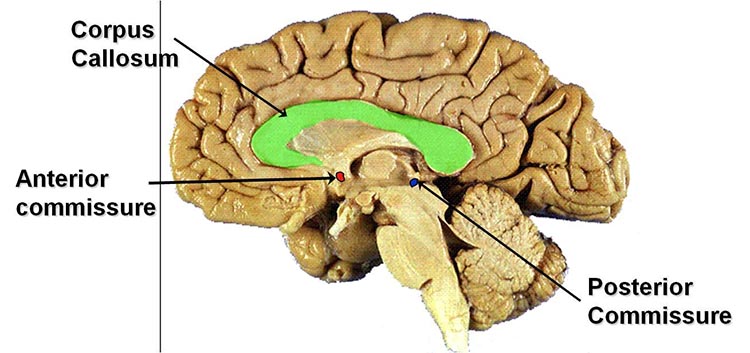
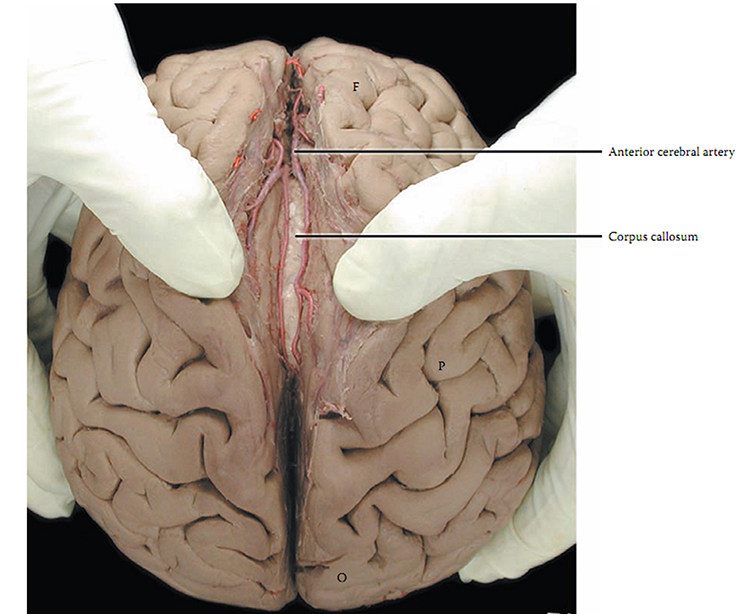
Subcortical Structures
Thalamus
The thalamus consists of specialized nuclei that process and relay data to and from the telencephalon (cerebral cortex, basal ganglia, and limbic system). The thalamus analyzes all sensory data except olfaction before distributing this information to the cortex via thalamocortical afferent fibers (Breedlove & Watson, 2020). The cortex also sends information to the thalamus to adjust its information processing via corticothalamic fibers. This two-way conversation creates feedback loops that are crucial generating several EEG rhythms. The thalamus helps regulate arousal, sleep, and wakefulness (Steriade & Llinás, 1988). Through its functional connection to the hippocampus, it plays a crucial role in episodic memory (Aggleton et al., 2010).The thalamus contributes to SCPs, delta, theta, alpha, SMR activity, and beta-gamma activity (Thompson & Thompson, 2016). Thalamus graphic © decade3d - anatomy online/Shutterstock.com.
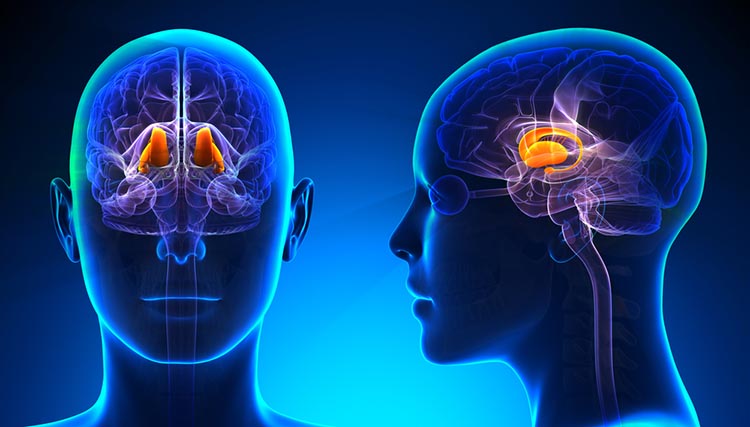
Basal Ganglia
The basal ganglia--the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra—-modulate movement. The basal ganglia, prefrontal cortex, cingulate cortex, and parietal cortex are involved in self-awareness, attention, and emotional regulation. Graphic © decade3d-anatomy online/Shutterstock.com.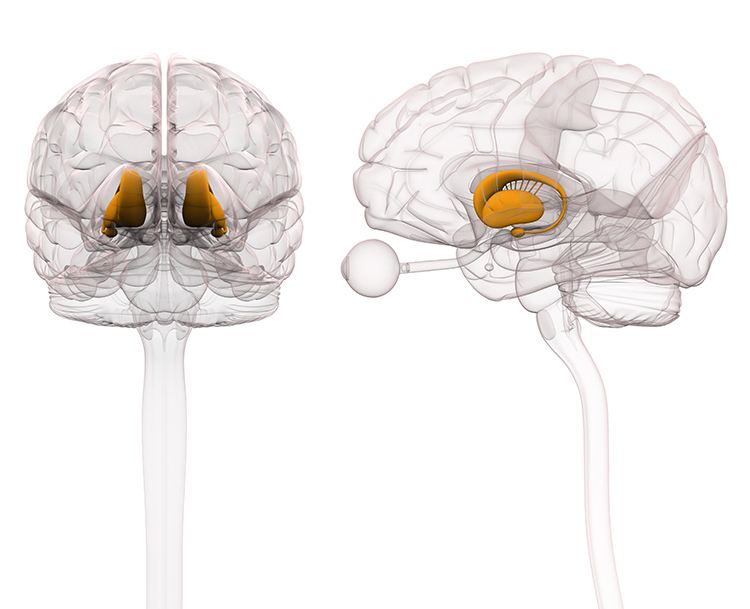
Limbic System
The limbic system is a poorly defined widespread network of nuclei involved in emotion, motivation, learning, memory, and navigation. Three important limbic structures are the hippocampus, amygdala, and septal nuclei (Breedlove & Watson, 2020). Limbic system graphic © joshya/Shutterstock.com.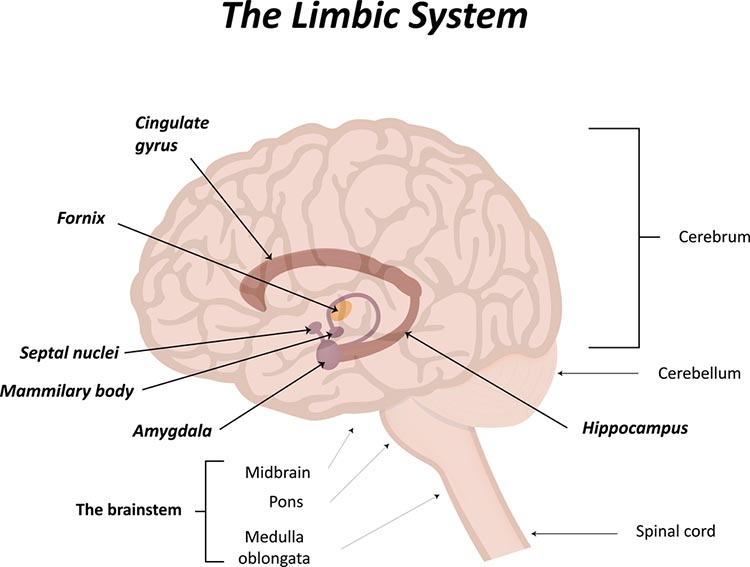
Hippocampus
The hippocampus is a seahorse-shaped limbic structure. The hippocampus is required to form declarative memories and plays a vital role in emotion, navigation, and spatial memory, and dampening the endocrine stress response. The hippocampus simultaneously integrates emotional, auditory, and visuospatial information to create episodic memories. The hippocampus also contains leukocyte receptors, making it part of the feedback loop for immune system regulation. Hippocampal neurons and networks that include it are sources of the theta rhythm (Amzica & Lopes da Silva, 2011).Hippocampal Brainwaves Travel in Two Directions
The old-school view was that hippocampal brainwaves travel in one direction. This model could not explain how the hippocampus integrates information from various interconnected specialized systems. The new-school view, based on recording from human participants undergoing brain surgery, is that brainwaves travel through the hippocampus in both directions: from the back to the front and from the front to the back (Kleen et al., 2021). Moreover, cognitive activity differentially influences the direction of movement for low (e.g., 1.9 Hz) and high (e.g., 13.8 Hz) frequency waveforms.Resources
Check out the Blausen Hippocampus animation. Human hippocampal neuron graphic © Kateryna Kon/Shutterstock.com.
Watch Sam Kean's TED-Ed Talk, What Happens When You Remove the Hippocampus. Hippocampus graphic © decade3d - anatomy online/Shutterstock.com.
Amygdala
The amygdala is an essential limbic structure located deep within the medial temporal lobes at the end of the hippocampus. The amygdala comprises many nuclei, including the lateral nucleus and the central nucleus. View the Blausen Amygdala animation.The lateral nucleus processes sensory information and distributes it throughout the amygdala. The central nucleus orchestrates the nervous system's response to important stimuli by activating circuits in the brainstem (autonomic arousal) and the basal ganglia, and periaqueductal gray (defensive behavior). The amygdala plays a crucial role in learning about the consequences of our actions and creating declarative memories for events with emotional significance (Breedlove & Watson, 2020). Amygdala graphic © decade3d - anatomy online/Shutterstock.com.
Septal Nuclei
The septal nuclei are a limbic structure that contains several nuclei involved in emotion, control of aggressive behavior, reward, and addiction (Breedlove & Watson, 2020). The septohippocampal system contributes to the theta rhythm (Amzica & Lopes da Silva, 2011). Septal nuclei graphic © 2016 Cheryl Cotman.
Brodmann Areas
Throughout the cortex, there are variations in the organization of cells and their axons and differences in cell anatomy, which can be used to define and differentiate areas of the cerebral cortex.The German anatomist Korbinian Brodmann produced cytoarchitectural maps of the cerebral cortex using Nissl staining, which outlines cell bodies. Brodmann divided the neocortex into 47 different numbered zones, each with its distinctive anatomy.
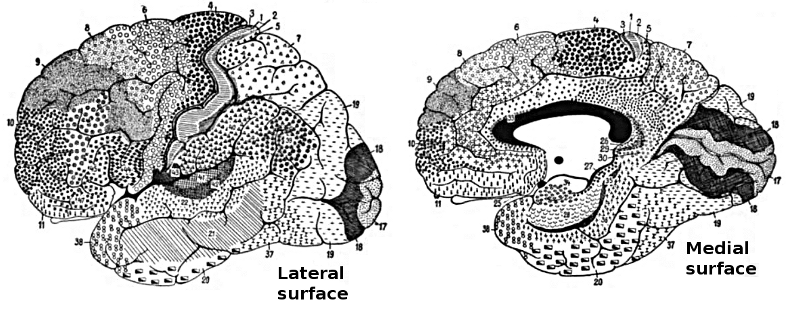
We continue to use these divisions. Graphic © 2008 Sinauer. Click on the Brodmann numbers on the graphic below to see corresponding Wikipedia articles.
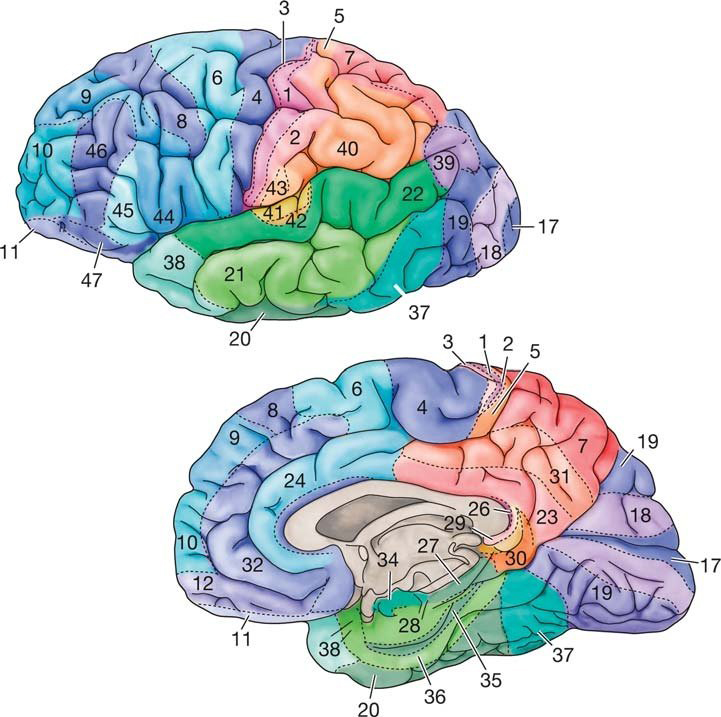
Human Brodmann Areas
Areas 3, 1, 2: Primary somatosensory cortexArea 4: Primary motor cortex
Area 5: Somatosensory association cortex
Area 6: supplementary motor cortex and premotor cortex
Area 7: Somatosensory association cortex
Area 8: Frontal eye fields
Area 9 and 46: Dorsolateral prefrontal cortex (DLPFC)
Area 10: Anterior prefrontal cortex
Areas 11 and 12: Orbitofrontal cortex
Areas 13 and 16: Insular cortex
Area 17: Primary visual cortex (V1)
Area 18: Secondary visual cortex (V2)
Area 19: Associative visual cortex (V3, V4, V5)
Area 20: Inferior temporal gyrus
Area 21: Middle temporal gyrus
Area 22: Superior temporal gyrus, including Wernicke's area
Area 23: Ventral posterior cingulate cortex
Area 24: Ventral anterior cingulate cortex
Area 25: Subgenual region of the ventromedial prefrontal cortex
Area 26: Ectosplenial region of the retrosplenial cerebral cortex
Area 27: Pyriform cortex
Area 28: Ventral entorhinal cortex
Area 29: Retrosplenial cingulate cortex
Area 30: Cingulate cortex
Area 31: Dorsal posterior cingulate cortex
Area 32: Dorsal anterior cingulate cortex
Area 33: Anterior cingulate cortex
Area 34: Dorsal entorhinal cortex
Areas 35 and 36: Perirhinal cortex
Area 37: Fusiform gyrus
Area 38: Temporopolar area
Area 39: Angular gyrus
Area 40: Supramarginal gyrus
Areas 41 and 42: Auditory cortex
Area 43: Primary gustatory cortex
Area 44: Pars opercularis (inferior temporal gyrus and part of Broca's area)
Area 45: Pars triangularis (inferior temporal gyrus and part of Broca's area)
Area 46: Dorsolateral prefrontal cortex (DLPFC)
Area 47: Pars orbitalis (part of inferior frontal gyrus)
Area 48: Retrosubicular area (small area of the medial temporal lobe)
Area 52: Parainsular area (junction of the temporal lobe and insula)
Source: Brodmann Area article in Wikipedia.
Researchers have revised the Brodmann maps and correlated areas with their functions. The Brodmann maps below were contributed by Mark Dow, Research Assistant at the Brain Development Lab, the University of Oregon to Wikimedia Commons.
CONNECTIVITY, PHASE, AND COHERENCE
Neural networks are systems of interconnected ensembles of neurons that collaborate to achieve a goal (Thompson & Thompson, 2016). Networks communicate and perform functions via hub- or node-based communication systems. Connectome graphic from van den Heuvel and Sporns (2011).
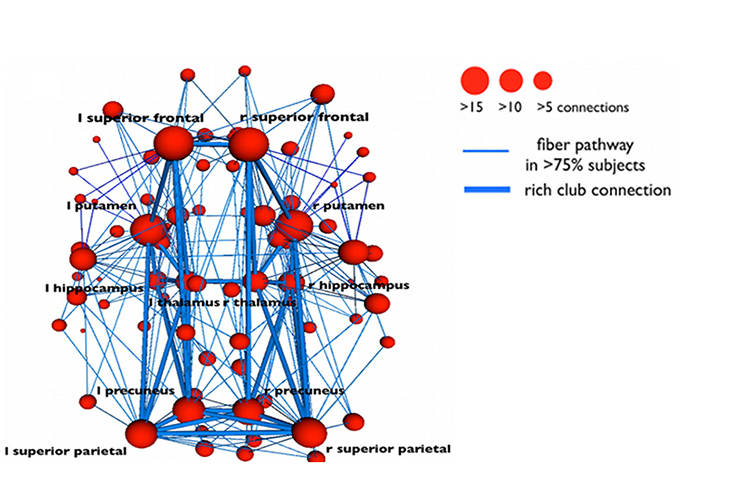
Connectivity
Networks like the Affect, Attention, Default, Executive, and Salience systems synchronize the activity of cortical and subcortical regions to perform functions. Connectivity is the degree of synchrony between the oscillations of specialized brain regions (nodes) within a network (Bastos & Schoffelen, 2016). Strongly connected brain regions are called hubs. Hubs can be primarily connected to nodes (vertices) within their local modules (sets of interconnected nodes) or nodes in more distant modules (Bullmore & Sporns, 2009).Neurofeedback training can increase or decrease connectivity using a normative database. For example, BrainMaster's BrainAvatar software allows clinicians to train specific networks, like the Default Mode Network (DMN).
See the Assumptions unit for an in-depth discussion of the Affective, Default Mode, Executive, Motor, Network, Oculomotor, Salience, and Social Networks.
Phase Reset Coordination of Neural Networks
Phase refers to the degree to which the peaks and valleys of EEG waveforms coincide. Phase measures the time shift between EEG activity in two brain regions. Phase represents the number of elements in a network times the delay in that network. The graphic below shows in-phase and out-of-phase waveforms.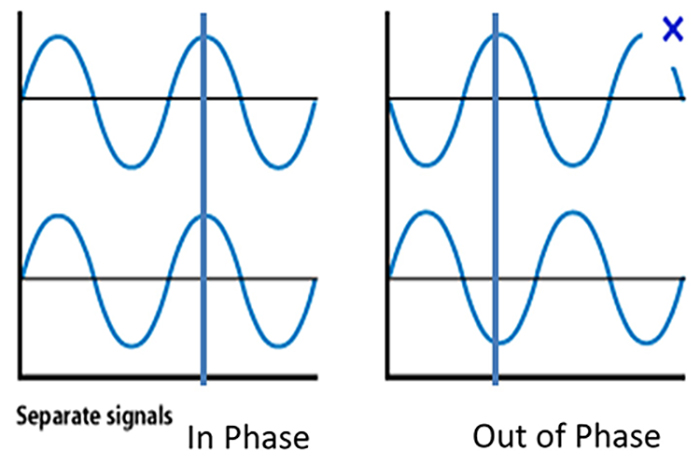
Phase reset (PR) is defined by a sudden change in phase difference (phase shift duration or SD) followed by a period of phase locking (lock duration or LD). PR = SD + LD (Thatcher et al., 2009). The graphic below shows multiple signals phase locking.
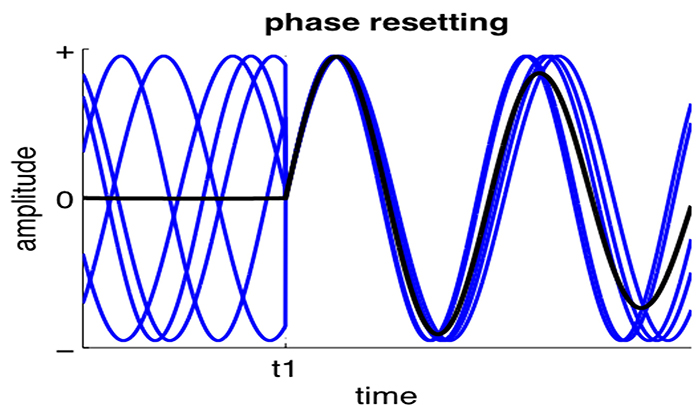
Gap junction coupling explains phase locking and phase shifting (Hughes & Crunelli, 2007).
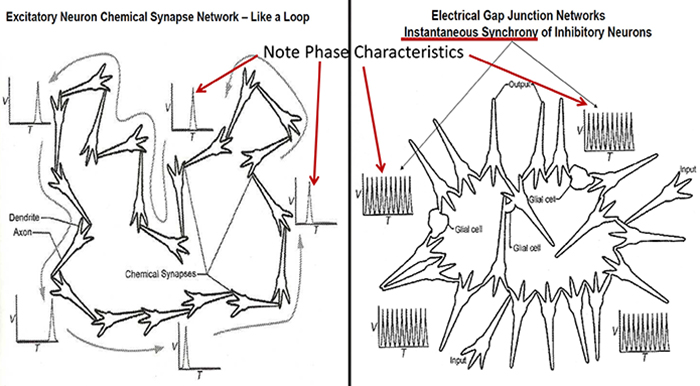
Resetting the phase of ongoing oscillatory activity to endogenous (internal) or exogenous (environmental) cues facilitates coordinated information transfer within circuits and between distributed brain areas. Phase resetting is a critical marker of dynamic state changes within functional networks (Voloh & Womelsdorf, 2016).
Phase rests create a neural context, a narrow band of frequencies that uniquely characterize the activated circuits. They impose coherent low-frequency phases to which high-frequency activations can synchronize. These are identifiable as cross-frequency correlations that span large distances. Phase rests are critical for neural coding models that depend on phase, increasing the informational content of neural representations. They likely originate from the dynamics of canonical E-I circuits that are anatomically ubiquitous.
Phase resets reorganize oscillations in diverse task contexts: attentional stimulus selection, classical conditioning, cross-modal integration, sensory perception, and spatial navigation. Phase resets can drive changes in ensemble organization, functional networks, neural excitability, and overt behavior.
The graphic below shows low- and high-frequency oscillations resulting from phase reset decreases and increases in neural activity, respectively.
EEG Synchrony and Phase
Networks of neurons generating the EEG activity at different sites can produce signals that are identical in amplitude, frequency, and phase or that are entirely unrelated.Synchrony means that the firing of pools of neurons is coordinated. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
Local synchrony occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals. For example, an alpha amplitude of 20-60 μV detected at O1-A1 is produced by the synchronous firing of pools of neurons. This site's beta activity amplitude is lower due to desynchronized firing. This is analogous to the volume generated by a choir. When performers sing in unison, they produce a louder sound than singing separately.
Frequency synchrony occurs when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
Phase synchrony occurs when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
Coherence
Coherence represents the degree of coupling between separate cortical regions and reflects neural network connectivity and dynamics (Bullmore & Sporns, 2009). Coherence evaluates the linear association or correlation between the EEG waveforms recording from two different scalp locations (two referential montages). A two-channel referential montage is shown below.
Coherence measures the degree to which two areas have consistent phase relationships at a designated frequency. When the phase difference between two signals is constant, coherence = 1. When the phase difference is random, coherence = 0.
Hyper-coherence means too much coupling and involves a failure to activate cortical regions selectively and may interfere with multitasking and rapid decision-making. Hypo-coherence, which often results from traumatic brain injuries, means too little coupling and involves a breakdown in communication between regions that should normally communicate with each other (Wilson et al., 2011).
Co-Modulation
Co-modulation is the degree of association in the magnitude of signals detected from two sources (sites). Co-modulation, which can be measured using the Pearson Product-Moment Correlation Coefficient, shows the degree to which signals strengthen and weaken in a correlated manner (Collura, 2009). Co-modulation does not measure phase or coherence, although it may reflect phase effects (Sterman & Kaiser, 2001).Glossary
acetylcholine: an amine neurotransmitter that binds to nicotinic and muscarinic ACh receptors.
acetylcholine esterase (AChE): the enzyme that deactivates ACh.
AChE-R: an abnormal form of acetylcholine esterase (AChE), which may render dendrites with acetylcholine receptors more excitable when stressed.
action potential: a propagated electrical signal that usually starts at a neuron’s axon hillock and travels to presynaptic axon terminals.
adenylate cyclase: at a metabotropic receptor, an enzyme that transforms ATP into the second messenger cyclic AMP.
afferent: a neuron that transmits sensory information towards the central nervous system, or from one region to another.
all-or-none law: once an action potential is triggered in an axon, it is propagated, without decrement, to the end of the axon. The amplitude of the action potential is unrelated to the intensity of the stimulus that triggers it.
alpha blocking: arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it entirely while increasing EEG power in the beta range.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: regular bursts of alpha activity.
alpha-subunit: a subunit of a G protein associated with the neuron membrane that breaks away to activate enzymes within the neuron when a ligand binds to a metabotropic receptor.
amino acid neurotransmitters: the oldest family of transmitters. These molecules bind to ionotropic and metabotropic receptors, transmitting information and modulating neuronal activity. Most synaptic communication is accomplished in the brain by glutamate (generally excitatory) and GABA (generally inhibitory).
AMPA (glutamate) receptors: ionotropic receptors which open sodium channels, depolarizing the neuron's membrane (producing an EPSP), and dislodging a Mg+ ion that blocks an adjacent NMDA (glutamate) receptor's calcium channel. AMPA receptors are responsible for most activity at glutamatergic synapses.
amplitude: the energy or power contained within the EEG signal measured in microvolts or picowatts.
amygdala: a limbic system structure plays a crucial role in learning about the consequences of our actions and creating declarative memories for events with emotional significance.
angular gyrus: the region located near the superior temporal lobe (BA 39) and involved in reading, math, and copying writing.
anion: negative ion, for example, chloride (Cl-).
anterior: near or toward the front of the head, for example, the anterior cingulate.
anterior cingulate cortex (ACC): a division of the prefrontal cortex (Fpz, Fz, Cz, Pz) that plays a vital role in attention and is activated during working memory. The ACC mediates emotional and physical pain and has cognitive (dorsal anterior cingulate) and affective (ventral anterior cingulate) conflict-monitoring components.
anterior commissure: a bundle of nerve fibers that crosses the midline and connects the left and right temporal lobes, hippocampus, and amygdala.
apical dendrite: a dendrite that arises from the top of the pyramid and extends vertically to layer 1 of the neocortex.
arousal: a process that combines alertness and wakefulness, produced by at least five neurotransmitters, including acetylcholine, histamine, hypocretin, norepinephrine, and serotonin.
astrocytes: star-shaped glial cells that communicate with and support neurons and help determine whether synapses will form.
asynchronous waves: voltages produced when neurons depolarize and hyperpolarize independently.
ATP: the energy source for a neuron’s sodium-potassium transporters.
auditory cortex: temporal cortex that processes auditory information within the dorsal and ventral streams.
autonomic nervous system: a subdivision of the peripheral nervous system that innervates glands and internal organ smooth muscles and includes enteric, parasympathetic, and sympathetic divisions.
autoreceptors: metabotropic receptors that can be located on the membrane of any part of a neuron. They detect neurotransmitters released the neuron releases, generate IPSPs that inhibit the neuron from reaching the excitation threshold, and regulate internal processes like transmitter synthesis and release through the second messenger system.
axoaxonic synapses: junctions between two axons that do not affect the generation of an action potential, only the amount of neurotransmitter distributed.
axodendritic synapses: junctions between axons and dendrites determine whether the axon hillock will initiate an action potential.
axon: long, cylindrical structures that convey information from the soma to the terminal buttons. An axon also transports molecules in both directions along the outer surface of protein bundles called microtubules.
axon hillock: a swelling in the cell body where a neuron integrates the messages it has received from other neurons and decides whether to fire an action potential.
axonal varicosity: a swelling in an axon wall allowing neurotransmitter release through the wall via volume transmission.
axoplasmic transport: the movement of molecules in both directions along the outer surface of protein bundles called microtubules.
basal dendrite: dendrite that horizontally branches out from the 30-μm base of the pyramid through the layer where the neuron resides.
basal forebrain: a cholinergic network located in the ventral frontal lobe and anterior hypothalamus that influences cerebral blood flow and cognitive activity.
basal ganglia: these forebrain structures consist of an egg-shaped nucleus that contains the putamen and globus pallidus and a tail-shaped structure called the caudate, which together are responsible for the production of movement. The basal ganglia have also been implicated in obsessive-compulsive disorder, Parkinson’s disease, and Huntington’s chorea.
benzodiazepine receptor agonist (BZRA) hypnotics: nonbenzodiazepines like zolpidem (Ambien).
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
bilateral synchronous slow waves: a pathological sign observed in drowsy children. When detected in alert adults, intermittent bursts of high amplitude slow waves may signify gray matter lesions in deep midline structures.
Broca's area: area located inferior frontal gyrus (BA 44 and 45) of the dominant hemisphere (F7-T3 in the left hemisphere) concerned with speech production, grammar, language comprehension, and sequencing.
Brodmann areas: 47 numbered cytoarchitectural zones of the cerebral cortex based on Nissl staining.
cation: a positive ion, for example, sodium (Na+).
caudal: away from the front of the head.
cell body or soma: contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
central nervous system (CNS): the division of the nervous system that includes the brain, spinal cord, and retina.
central nucleus of the amygdala: the nucleus that orchestrates the nervous system's response to essential stimuli by activating circuits in the brainstem (autonomic arousal) and the basal ganglia and periaqueductal gray (defensive behavior).
central sulcus: fissure that separates the frontal and parietal lobes.
central nucleus of the amygdala: nucleus that orchestrates the nervous system's response to important stimuli by activating circuits in the brainstem (autonomic arousal) and the basal ganglia and periaqueductal gray (defensive behavior).
cerebral cortex: the layer of gray matter that covers the cerebral hemispheres. The cerebral cortex consists of gray matter and white matter.
cerebral ventricles: a network of fluid-filled chambers that protects the brain from trauma due to abrupt head movements and facilitates the exchange of nutrients and wastes between blood vessels and the brain.
cerebrospinal fluid (CSF): fluid produced by the choroid plexus membrane of the lateral ventricles that fills the ventricular system.
chemical synapses: junctions between neurons that transmit molecules across gaps of less than 300 angstroms. Neurons use chemical synapses to produce short-duration (milliseconds) and long-duration (seconds to hours) changes in the nervous system. Chemical synapses are capable of more extensive communication and initiate more diverse and long-lasting changes than electrical synapses.
circle of Willis: vascular network located at the base of the brain comprised of the carotid and basilar arteries. This structure may provide another route for delivering blood when a major artery is compromised by disease or traumatic injury.
classical routes for EEG activation: specific sensory pathways like the visual (retina to the visual cortex), auditory (cochlea to the auditory cortex), and somatosensory (chemoreceptors and mechanoreceptors to the somatosensory cortex) systems. Increased transmission of information through these pathways desynchronizes EEG activity in the cortical regions to which these afferent neurons project, as specialized circuits of neurons independently process this information.
coherence: the degree of coupling between separate cortical regions and reflects neural network connectivity and dynamics. Coherence evaluates the linear association or correlation between the EEG waveforms recording from two different scalp locations (two referential montages).
commissures: axon tracts. The left and right hemispheres communicate using the corpus callosum, anterior commissure, and posterior commissure.
co-modulation: the degree of association in the magnitude of signals detected from two different sources (sites). Co-modulation, which can be measured using the Pearson Product-Moment Correlation Coefficient, shows the degree to which signals strengthen and weak in a correlated manner.
complex: a sequence of waves.
COMT: a degrading enzyme that only targets the catecholamines dopamine and norepinephrine.
connectivity: the degree of synchrony between the oscillations of specialized brain regions (nodes) within a network.
contingent negative variation (CNV): a steady, negative shift in potential (15 μV in young adults) detected at the vertex. This slow cortical potential may reflect expectancy, motivation, intention to act, or attention. The CNV appears 200-400 ms after a warning signal (S1), peaks within 400-900 ms, and sharply declines after a second stimulus that requires the performance of a response (S2).
continuous irregular delta: slow waves produced by white matter lesions seen in disorders like multiple sclerosis.
contralateral: structures that are located on opposite sides of the body. For example, neurons in the left primary motor cortex control muscles on the right side of the body.
coronal plane: the plane that separates the body into front and back parts.
corpus callosum: the largest commissure that connects the left and right frontal, parietal, and occipital lobes.
corticothalamic network: a unified network that generates diverse types of brain rhythms grouped by slow cortical oscillations.
cranial nerves: 12 pairs of nerves connected to the brain and are part of the sensory and motor systems of the head and neck.
cyclic AMP: a second messenger that moves about the neuron, activating other enzymes. Protein kinase A, which controls the excitability of ion channels, is a crucial enzyme target of cyclic AMP. Cyclic AMP also travels to the nucleus to regulate gene expression.
Dale's principle: the incorrect view that a neuron can only release one neurotransmitter. They often release two to four.
delta rhythm: 0.05-3-Hz oscillations generated by thalamocortical neurons during stage-3 sleep.
dendrite: a branched structure designed to receive messages from other neurons via axodendritic synapses (junctions between axons and dendrites), determining whether the axon hillock will initiate an action potential.
dendritic spines: protrusions on the dendrite shaft where axons typically form axodendritic synapses.
dendrodendritic synapses: junctions between dendrites that communicate chemically across synapses and electrically across gap junctions.
depolarize: to make the membrane potential more positive by making the inside of the neuron more positive with respect to its outside.
desynchronization: the absence or loss of coordinated neuronal firing and synchronization of brain waves.
diencephalon: the posterior forebrain subdivision that contains the thalamus and hypothalamus.
diffusion: the distribution of molecules from areas of high concentration to low concentration.
diphasic wave: a wave that contains both a negative and positive deflection from the baseline.
dipole: the electrical field generated between the sink (where current enters the neuron) and the source (place at the other end of the neuron where current leaves) may be located anywhere along the dendrite.
distal: toward the periphery.
dominant frequency: EEG frequency with the greatest amplitude.
dopamine: a monoamine neurotransmitter that exerts its postsynaptic effects on at least six receptors linked to G proteins. This means that dopamine functions as a neuromodulator. The two major families include D1 (D1 and D5) and D2 (D2A, D2B, D3, and D4).
dorsal: toward the upper back or head.
dorsal stream (auditory): the pathway from the temporal to the parietal lobes that helps spatially localize sounds.
dorsal stream (visual): the pathway from the primary visual cortex (V1) to the parietal lobe that helps to localize objects and guide movements towards them.
dorsolateral prefrontal cortex (DLPFC): the region of the middle frontal gyrus (BA 9 and 4) that shares responsibility with cortical and subcortical networks for executive functions like abstract reasoning, cognitive flexibility, decision-making, inhibition, planning, and working memory (Miller & Cummings, 2007) and exercises the highest cortical level of motor control.
D-serine: a neurotransmitter that binds to the glycine site on the NMDA receptor to trigger calcium entry into a dendritic spine when glutamate binds to its site, resulting in a large, prolonged increase in intracellular calcium.
dual-action antidepressants: medications that activate 5-HT1 receptors to produce antidepressant and anxiolytic effects, while they blockade 5-HT2 (agitation, restlessness, and sexual dysfunction) and 5-HT3 (nausea, headache, and vomiting) receptors to minimize their side effects.
EEG activity: a single wave or successive waves.
EEG power: the signal energy in the EEG spectrum. Most EEG power falls within the 0-20 Hz frequency range. EEG power is measured in microvolts or picowatts.
efferent: a motoneuron that transmits information towards the periphery.
electrical synapse: a symmetrical synapse where neurons communicate information bidirectionally across gap junctions between adjacent membranes using ions. Transmission across electrical synapses is instantaneous, compared with the 10 ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of distant neurons to synchronize their activity and simultaneously fire.
electroencephalogram (EEG): the voltage difference between at least two electrodes, where at least one electrode is located on the scalp or inside the brain. The EEG is a recording of both EPSPs and IPSPs that occur primarily in dendrites in pyramidal cells located in macrocolumns, several mm in diameter, in the upper cortical layers
electrostatic pressure: the attractive or repulsive force between ions that moves them from one region to another.
entorhinal cortex: a structure located in the caudal region of the temporal lobe and that receives pre-processed sensory information from all modalities and reports on cognitive operations. The entorhinal cortex provides the main input to the hippocampus, is involved in memory consolidation and spatial localization, and provides input into the septohippocampal system that may generate the 4-7 Hz theta rhythm.
enzymatic deactivation: the process in which an enzyme breaks a neurotransmitter apart into inactive fragments. For example, acetylcholine transmission is ended by the enzyme acetylcholine esterase (AChE). Deactivating enzymes located in the synaptic cleft degrade a neurotransmitter molecule when it detaches from its binding site.
evoked potential: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual). An evoked potential has a negative peak at 80-90 ms and a positive peak around 170 ms following stimulus onset. The orienting response ("What is it?") is a sensory ERP. The N1-P2 complex in the auditory cortex of the temporal cortex reveals whether an uncommunicative person can hear a stimulus.
excitatory postsynaptic potential (EPSP): a brief positive shift in a postsynaptic neuron's potential produced when neurotransmitters bind to receptors and cause positive sodium ions to enter the cell. An EPSP pushes the neuron towards the excitation threshold when it can initiate an action potential.
exocytosis: the process of neurotransmitter release. When an action potential arrives and depolarizes the terminal button, calcium ions enter the terminal button from the extracellular fluid. Calcium binds with clusters of protein molecules that join the vesicles with the presynaptic membrane. The clusters move apart, forming a hole through both membranes called a fusion pore, and the neurotransmitter leaves the terminal button for the synaptic cleft or extracellular fluid.
exogenous ERP: an event-related potential (ERP) elicited by external sensory stimuli (auditory, olfactory, somatosensory, and visual).
explicit learning: behavioral changes that occur with our conscious awareness that require processing by the hippocampus.
extracellular dipole layers: macrocolumns of pyramidal cells, which lie parallel to the surface of the cortex, send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons.
extracellular fluid: the fluid surrounding a neuron.
facultative pacemaker theory: Anderson and Anderson's (1968) theory that thalamic neurons activate cortical neurons and thalamic inhibitory interneurons via recurrent collaterals.
fast cortical potentials: EEG rhythms that range from 0.5 Hz-100 Hz. The main frequency ranges include delta, theta, alpha, sensorimotor rhythm, and beta.
feature binding: the process of linking information to perceptual objects (linking an apple's color to its shape) that may involve the 40-Hz rhythm.
fissures: deep grooves, for example, the lateral fissure.
focal waves: EEG waves detected within a limited area of the scalp, cerebral cortex, or brain.
forebrain: the anterior brain subdivision that consists of the cerebral hemispheres (telencephalon) and the thalamus and hypothalamus (diencephalon), also called the prosencephalon.
frequency: the number of cycles completed each second expressed in hertz (Hz).
frequency synchrony: when identical EEG frequencies are detected at two or more electrode sites. For example, 12 Hz may be simultaneously detected at O1-A1 and O2-A2.
frontal lobes: the most anterior cortical lobes of the brain (F7, F3, Fz, F8, F4) that are divided into the primary motor cortex, motor association cortex, Broca's area, and prefrontal cortex.
fusion pore: a hole through a vesicle and presynaptic membrane that allows neurotransmitters to leave the terminal button for the synaptic cleft or extracellular fluid.
G protein: a protein located inside a neuron’s membrane next to a metabotropic receptor, activated when the receptor binds a ligand. An alpha-subunit of the G protein then breaks away to perform actions within the cell.
GABA: an amino acid that is often inhibitory. GABA may be the most important inhibitory neurotransmitter in the brain. There are several types of GABA receptors, each producing inhibition differently.
gamma rhythms: a 28-80 Hz rhythm that includes the 38-42 Hz Sheer rhythm and is associated with learning and problem-solving, meditation, mental acuity, and peak brain function in children and adults.
gap junction: an electrical synapse, which is a symmetrical synapse where neurons communicate information bidirectionally across gap junctions between adjacent membranes using ions. Transmission across electrical synapses is instantaneous, compared with the 10-ms or longer delay in chemical synapses. The rapid information transmission that characterizes electrical synapses enables large circuits of distant neurons to synchronize their activity and simultaneously fire.
generalized asynchronous slow waves: waves seen in sleepy children and those with elevated temperatures. This may indicate degenerative disease, dementia, encephalopathy, head injury, high fever, migraine, and Parkinson's disease in adults.
glial cells: nonneural cells that guide, insulate, and repair neurons and provide structural, nutritional, and information-processing support. Glial cells generate slow cortical potentials (SCPs). Glial cells include astrocytes, microglia, oligodendrocytes, radial glial cells, and Schwann cells.
global loops: cortical macrocolumns separated by as much as 7 cm and receive shared input fire synchronously to generate delta and theta rhythms.
glutamate: an amino acid that is often excitatory and that may be the primary excitatory neurotransmitter in the brain. Its receptors are found on the surface of almost all neurons. There are at least 13 different receptors for glutamate, 5 ionotropic and 8 metabotropic. Most presynaptic neurons in the brain excite postsynaptic neurons via ionotropic glutamate receptors in the postsynaptic membrane. Metabotropic glutamate receptors may play a regulatory function, either augmenting or suppressing the activation of ionotropic glutamate receptors.
glycine: an amino acid that is often inhibitory and has a binding site on the NMDA receptor.
gray matter: brain tissue that looks grayish brown and comprises cell bodies, dendrites, unmyelinated axons, glial cells, and capillaries.
gyrus: ridge of cortex demarcated by sulci or fissures, for example, the precentral gyrus.
hertz (Hz): unit of frequency, an abbreviation for cycles per second.
high alpha (alpha 2): 10-12-Hz alpha associated with open awareness.
high beta (beta 4): 25-38-Hz activity mostly seen in the frontal lobes and is associated with hyper-perfusion and increased glucose metabolism. High or fast beta activity may be related to peak performance and cognitive processing and related to specificity and precision in information processing. Excessive high beta is associated with alcoholism, anxiety, OCD, rumination, and worry.
hindbrain: posterior brain division that consists of the cerebellum, pons, and medulla.
hippocampus: a seahorse-shaped limbic structure. The hippocampus is required to form declarative memories and plays a vital role in emotion, navigation, spatial memory, and dampening the endocrine stress response. The hippocampus also contains leukocyte receptors, making it part of the feedback loop for immune system regulation. Hippocampal neurons and networks that include it are sources of the theta rhythm.
horizontal (transverse) plane: the plane that divides the brain into upper and lower parts.
hubs: highly centralized nodes through which other node pairs communicate; hubs allow efficient communication.
hyper-coherence: excessive coupling due to a failure to selectively activate cortical regions. Hyper-coherence may interfere with multitasking and rapid decision-making.
hypo-coherence: deficient coupling due to a breakdown in communication between regions that should generally communicate with each other. Hypo-coherence often results from traumatic brain injuries.
hyperpolarize: a negative shift in membrane potential (the inside becomes more negative with respect to the outside) due to the loss of positive ions or gain of negative ions.
inferior colliculi: midbrain structures that integrate information about spatial localization and multiple sensory modalities, including somatosensory information.
inhibitory postsynaptic potential (IPSP): a brief negative shift in a postsynaptic neuron's potential produced when cations like potassium leave a neuron or anions (negative ions) like chloride enter a neuron, which hyperpolarizes the cell. An IPSP pushes the neuron away from the threshold of excitation.
insular cortex: cortex that lies deep within the lateral sulcus that divides the temporal and parietal lobes (BA 13). The insula is involved in emotional and autonomic responses to external stimuli and is part of the salience network.
integration: the addition of EPSPs and IPSPs at the axon hillock. Neurons sum EPSPs and IPSPs over their surface in spatial integration and over ms of time in temporal integration to raise the membrane from its resting potential to the excitation threshold. EPSPs and IPSPs last from 15-200 ms, while action potentials occur in 1-2 ms.
internal carotid artery: a major paired artery that supplies blood to nearly two-thirds of the cerebral hemispheres.
interneurons: neurons that receive input from and distribute output to other neurons. They have short processes and are confined to the central nervous system. They provide the integration required for decisions, learning and memory, perception, planning, and movement.
intracellular fluid: the watery cytoplasm contained within a neuron.
ion: a charged atom or molecule with a positive or negative charge. Positive ions are called cations, and negative ions are called anions.
ionotropic receptor: receptor protein that contains a binding site for a ligand and an ion channel that opens when the neurotransmitter attaches to this site.
ipsilateral: structures that are located on the same side of the body. For example, the left olfactory bulb distributes axons to the left hemisphere.
irregular waves: successive waves that constantly alter their shape and duration.
kappa rhythm: bursts of alpha or theta and is detected over the temporal lobes of subjects during cognitive activity.
lambda waves: saw-toothed transient waves from 20-50 mV in amplitude and 100-250 ms in duration detected over the occipital cortex during wakefulness. These positive deflections are time-locked to saccadic movements and observed during visual scanning, as during reading.
lateral: to the side, away from the center, as in the lateral geniculate nucleus.
lateral geniculate nucleus (LGN): thalamic nucleus that relays visual information to the cortex.
lateral nucleus of the amygdala: a nucleus that processes sensory information and distributes it throughout the amygdala.
lateralized waves: waves that are primarily detected on one side of the scalp and may indicate pathology.
Layers I-III: cortical layers that receive corticocortical afferent fibers that connect the left and right hemispheres.
Layer III: the cortical layer that is the primary source of efferent corticocortical fibers.
Layer IV: the cortical layer that is the primary destination of thalamocortical afferents and intra-hemispheric corticocortical afferents.
Layer V: the cortical layer that is the primary origin of efferent fibers that target subcortical structures that have motor functions.
Layer VI: the cortical layer that projects cortico-thalamic efferent fibers to the thalamus, which, together with the thalamocortical afferents, creates a dynamic and reciprocal relationship between these two structures.
left dorsolateral prefrontal cortex: the division of the prefrontal cortex concerned with approach behavior and positive affect. It helps us select positive goals and organizes and implements behavior to achieve these goals.
limbic system: a poorly-defined widespread network of nuclei involved in emotion, motivation, learning, memory, and navigation. Three important limbic structures are the hippocampus, amygdala, and septal nuclei.
local loops: neighboring cortical macrocolumns that share input generate frequencies above 30 Hz in the high-beta and gamma ranges.
local synchrony: synchrony that occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals.
localized slow waves: waves that may indicate a transient ischemic attack (TIA) or stroke, migraine, mild head injury, or tumors above the tentorium. Deep lesions result in bilateral or unilateral delta.
locus coeruleus system: the noradrenergic branch of the ascending reticular activating system that projects to the thalamus, limbic system, and cerebral cortex, and contributes to wakefulness and vigilance for salient stimuli. Subnormal norepinephrine transmission may contribute to ADHD.
long-latency potentials: potentials that have extended latencies following stimulus onset, for example, P300 and N400 ERPs.
long-term depression (LTD): a persistent decrease in synaptic strength following low-frequency stimulation.
long-term potentiation (LTP): a persistent increase in synaptic strength following high-frequency stimulation.
low alpha (alpha 1): 8-10-Hz alpha below a client's peak alpha frequency when eyes are closed.
macrocolumns: circuits of cortical pyramidal neurons several millimeters in diameter that create extracellular dipole layers parallel to the surface of the cortex, that send opposite charges towards the surface and the deepest of the 5-7 layers of cortical neurons. Since the pyramidal neurons are all aligned with the cortical surface, the postsynaptic potentials at cells within the same macrocolumn add together because they have the same positive or negative charge. The macrocolumns fire synchronously.
medial: toward the center of the body, away from the side. For example, the medial geniculate nucleus.
medial geniculate nucleus (MGN): thalamic nucleus that projects to several cortical auditory areas using two separate pathways. The MGN mainly relays frequency, amplitude, and binaural information to the auditory cortex in the temporal lobe.
medial prefrontal cortex: the division of the prefrontal cortex that integrates cognitive-affective information and helps control the hypothalamic–pituitary–adrenal (HPA) axis during emotional stress.
membrane potential: a neuron’s electrical charge created by a difference in ion distribution within and outside the neuron. A typical resting potential is about -70 mV (thousandths of a volt) since the inside of a resting axon is more negatively charged than the outside.
meninges: three protective layers (dura mater, pia mater, and arachnoid) that enclose the brain and spinal cord.
mesocortical neurons: dopaminergic neurons that project from the ventral tegmental area of the midbrain to the prefrontal cortex and excite prefrontal cortical neurons that control working memory, planning, and strategy preparation for problem solving. Underactivity in this pathway is associated with the negative symptoms of schizophrenia-like attentional deficits.
metabotropic receptors: include all G protein-linked receptors located on neurons, including autoreceptors. Neurotransmitters that bind to G protein-linked receptors are often called neuromodulators. Metabotropic receptors, which indirectly control the cell's operations, expend energy, and produce slower, longer-lasting, and more diverse changes than ionotropic receptors. Their effects can last several seconds, instead of milliseconds, because of the long-lived activity of G proteins and cyclic AMP.
metencephalon: the hindbrain subdivision that consists of the cerebellum and pons.
microtubules: hollow cylindrical protein bundles that are involved in axoplasmic transport.
midbrain: the middle division called the mesencephalon, which includes the inferior colliculi, superior colliculi, and substantia nigra.
mirror neurons: neurons activated when we perform a movement or observe others perform the same activity. Mirror neurons may facilitate observational learning, understanding others' actions and intentions, and empathy.
modulating effects: neuromodulators like the monoamines alter the performance of diffuse networks of target neurons by indirectly controlling cellular operations when they bind to metabotropic receptors.
module: a set of interconnected nodes in a neural network.
monoamine neurotransmitters: amine neurotransmitters that include dopamine, norepinephrine, epinephrine (catecholamines), and serotonin (indoleamine). These neurotransmitters are released using volume transmission and generally have modulating effects, altering the performance of diffuse networks of target neurons.
monoamine oxidase (MAO): an enzyme that degrades and inactivates the monoamine neurotransmitters dopamine, norepinephrine, and serotonin.
monoamine oxidase inhibitors (MAOIs): antidepressant drugs that interfere with MAO's breakdown of monoamines and increase monoamine availability to treat clinical depression.
monophasic wave: either a single negative (upward) or positive (downward) deflection from baseline.
motor cortex: a subdivision of the frontal lobe located in the precentral gyrus and guides fine motor coordination (like writing).
motor ERPs: event-related potentials detected over the primary motor cortex (precentral gyrus) during movement. Their amplitude is proportional to the force and rate of skeletal muscle contraction.
motor nerves: efferent neurons that convey commands to glands, muscles, and other neurons.
movement-related potentials (MRPs): slow cortical potentials that occur at 1 second as subjects prepare for unilateral voluntary movements. MRPs are distributed bilaterally with maximum amplitude at Cz. The supplementary motor area and primary motor and somatosensory cortices primarily generate these potentials.
mu rhythm: 7-11-Hz waves resemble wickets and appear as several-second trains over central or centroparietal sites (C3 and C4).
multiple spike-and-slow-wave complex: multiple spikes associated with at least one slow wave.
muscarinic receptors: metabotropic ACh receptors that are stimulated by muscarine and blocked by atropine. Muscarinic receptors control smooth muscle and predominate in the CNS. In the CNS, muscarinic receptors help mediate learning, memory, attention, arousal, EEG, and postural control.
myelencephalon: the hindbrain subdivision that consists of the medulla.
myelinated axons: axons insulated by myelin by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system.
N1-P2: a sensory event-related potential in the auditory cortex of the temporal cortex that reveals whether an uncommunicative person can hear a stimulus.
N400 potential: an event-related potential (ERP) elicited when we encounter semantic violations like ending a sentence with a semantically incongruent word ("The handsome prince married the beautiful fish"), or when the second word of a pair is unrelated to the first (BATTLE/GIRL).
negative SCPs: slow cortical potentials produced by glial cells that increase the probability of neuron firing.
nerve: bundled axons outside of the central nervous system.
neural network: a system of interconnected ensembles of neurons that collaborate to achieve a goal. These networks communicate and perform functions via hub- or node-based communication systems.
neuroaxis: an imaginary line that runs centrally through the central nervous system (CNS) from the front of the prefrontal cortex to the base of the spinal cord.
neuromodulator: neurochemical that modifies the effect of neurotransmitters through mechanisms like binding to metabotropic receptors.
neuron: a nerve cell that is the fundamental anatomical unit of the nervous system.
nicotinic ACh receptor: an ionotropic receptor that is stimulated by nicotine and blocked by curare. They are mainly found in the PNS on skeletal muscles. At CNS axoaxonic synapses, they produce presynaptic facilitation (increase neurotransmitter release). In the CNS, nicotinic receptors help regulate cortical blood flow, anxiety reduction, and decision-making.
nigrostriatal pathway: a dopaminergic pathway from the substantia nigra to the basal ganglia (caudate nucleus and putamen) that controls movement. The nigrostriatal pathway is progressively destroyed in Parkinson’s disease.
nitric oxide: a gaseous retrograde transmitter that is involved in long-term potentiation (LTP).
NMDA (glutamate) receptors: ligand-gated and voltage-gated glutamate receptors that bind the glutamate agonist NMDA. NMDA receptors play an important role in long-term potentiation (LTP).
node: a vertex within a neural network.
nodes of Ranvier: gaps between myelinated axon segments where the axon membrane is exposed to extracellular fluid and action potentials are regenerated by sodium ion entry.
norepinephrine: a monoamine neurotransmitter that exerts postsynaptic effects at alpha and beta receptors, each with two subtypes. All norepinephrine receptors are G protein-linked. The cell bodies of the most critical noradrenergic system are located in the locus coeruleus, a nucleus found in the dorsal pons.
nucleus accumbens: a limbic structure that receives dopamine released by the mesolimbic pathway. The nucleus accumbens plays a critical role in reinforcing diverse activities, including ingestion of drugs like central nervous system stimulants.
nucleus reticularis: a thalamic nucleus that may function as a pacemaker by releasing the inhibitory transmitter GABA at synapses with thalamocortical neurons.
occipital lobes: cortical lobes (Oz, O1, O2) posterior to the parietal lobes. The primary visual cortex (VI) is located within the calcarine sulcus (BA 17). They process visual information from the eyes in collaboration with the frontal, parietal, and temporal lobes.
odd-ball stimulus: a meaningful stimulus that is different from others in a series used to elicit the P300 potential. For example, a colored playing card is presented in a series of monochrome cards.
open awareness: the ability to adaptively respond to various environmental changes.
orbitofrontal cortex (OFC): the frontal lobe subdivision (BA 10, 11, and 47) that may aid planning by evaluating the consequences (rewards and punishments) of our actions and helping to generate the motivation to ingest drugs. The OFC appears to adjust decision-making based on the stakes involved and enables us to switch between substantial (investments) and trivial (snacks) choices.
orienting response: Pavlov’s "What is it?" reaction to stimuli like the sound of a vase crashing that includes (1) increased sensory sensitivity, (2) head (and ear) turning toward the stimulus, (3) increased muscle tone (reduced movement), (4) EEG desynchrony, (5) peripheral constriction and cephalic vasodilation, (6) a rise in skin conductance, (7) heart rate slowing, and (8) slower, deeper breathing.
P300 potential: an event-related potential (ERP) with a 300-900 ms latency and greatest positive peaks located over parietal lobe sites. The P300 potential may reflect an event’s subjective probability, meaning, and transmission of information.
parahippocampal gyri: structures located within the medial temporal lobe that form spatial and nonspatial contextual associations, which serve as building blocks for contextual processing, episodic memory, navigation, and scene processing. They may also play a role in emotional responsiveness.
parietal lobes: cortical lobes (Pz, P3, P4) posterior to the frontal lobes divided into the primary somatosensory cortex (postcentral gyrus) and secondary somatosensory cortex. Their primary function is to process somatosensory information like pain and touch.
perception-action cycles: cognitive and emotional processes that adapt (and preadapt) us to our environment.
peripheral nervous system (PNS): autonomic and somatic nervous system neurons and nerves outside the skull and spinal cord.
phase: the degree to which the peaks and valleys of EEG waveforms coincide. Phase measures the time shift between EEG activity in two brain regions.
phase reset: a sudden change in phase difference (phase shift duration or SD) followed by a period of phase locking (lock duration or LD). PR = SD + LDs.
phase synchrony: synchrony when identical EEG frequencies are detected at two or more electrode sites, and the peaks and valleys of the EEG waveforms coincide. This is also called global synchrony. For example, EEG training may produce phase-synchronous 12-Hz alpha waves at O1-A1 and O2-A2.
polyphasic (multiphasic) wave: a wave that contains two or more deflections of opposite polarity from baseline.
positive SCPs: slow cortical potentials produced by glial cells that decrease the probability of neuron firing.
postcentral gyrus: primary somatosensory cortex, posterior to the central sulcus.
posterior: near or toward the back of the head.
posterior basic rhythm: posterior alpha rhythm associated with IQ and memory performance.
posterior cerebral arteries: the left and right posterior arterial branches of the basilar artery that supply blood to the posterior cerebral hemispheres, cerebellum, and brainstem.
posterior commissure: axon tracts located below the corpus callosum connect the right and left diencephalon and mesencephalon.
posterior cortex: parietal, temporal, and occipital cortical areas concerned with perception and memory.
precentral gyrus: primary motor cortex, anterior to the central sulcus.
prefrontal cortex (PFC): the most anterior frontal lobe division (BA 9, 10, 11, 12, 46, 47) that is subdivided into dorsolateral, medial, orbitofrontal, and anterior cingulate regions and is responsible for executive functions like attention, working memory, prediction of the outcomes of current and hypothetical actions, the ability to work toward goals, problem-solving, planning, and the ability to suppress actions that could lead to unwanted outcomes.
premotor cortex (motor association cortex): the frontal lobe subdivision (BA 6) that is anterior to the motor cortex and helps to program and execute head, trunk, and limb movements.
presynaptic facilitation: a modulatory process in which a neuron increases the presynaptic neuron's neurotransmitter release by delivering a neurotransmitter that increases calcium ion entry into its terminal button.
presynaptic inhibition: a modulatory process in which a neuron decreases neurotransmitter release by reducing calcium ion entry.
primary motor cortex: the frontal lobe region located along the precentral gyrus (BA 4) that organizes the opposite side of the body's muscles and movements required for fine motor coordination in tasks like writing. Lesions can result in loss of motor control, including rigid paralysis.
primary somatosensory cortex (S1): parietal lobe subdivision located in the parietal lobe's postcentral gyrus posterior to the central sulcus (BA 3, 1, and 2). S1 maps touch and pain information from the opposite side of the body.
primary visual cortex (V1): the occipital lobe region (also called striate cortex) which receives most visual information from the lateral geniculate nucleus of the thalamus.
protein kinase A: an intracellular enzyme that controls the excitability of ion channels and is a vital enzyme target of cyclic AMP.
raphe system: the midline network of cell bodies within the brainstem and midbrain that may influence alertness and vigilance through reciprocal connections with the suprachiasmatic nucleus of the hypothalamus.
rate law: the principle that neurons represent the intensity of a stimulus by variation in the rate of axon firing.
readiness potential: slow-rising, negative potential (10-15 μV) detected at the vertex before voluntary and spontaneous movement. This slow cortical potential precedes voluntary action by 0.5 to 1 s and peaks when the subject responds.
regional loops: cortical macrocolumns that share input and are separated by several centimeters generate alpha and beta rhythms.
regular or monomorphic waves: successive waves with identical shapes. Regular waves may resemble sine waves (sinusoidal) or maybe arched (resembling wickets), or saw-toothed (asymmetrical and triangular).
resonant loop: the synchronous firing by macrocolumns that share afferent input to generate an electrical potential.
resting potential: the membrane potential of a neuron when it is not influenced by messages from other neurons.
reticular activating system (RAS): a network of 90 nuclei within the central brainstem from the lower medulla to the upper midbrain. The reticular formation sends axons to the spinal cord, thalamus, and cortex, contributing to diverse functions like neurological reflexes, muscle tone and movement, attention, arousal, and sleep.
reuptake: the primary method that neurons terminate the action of neurotransmitters. Reuptake transporters located in terminal buttons and astrocytes remove neurotransmitters from the synaptic cleft.
reward deficiency syndrome: Blum’s hypothesis that an abnormal form of the A1 allele is present in most severe alcoholics and results in defective D2 receptors. Reduced D2 receptor activity may reduce the activation of the nucleus accumbens and hypothalamus and result in dysphoria, drug craving, and compulsive drug-seeking and abuse.
rhythmic slow wave activity: a posterior waveform generated by the limbic system and thalamus that is mostly seen in the frontal-midline (FCz) when awake with eyes open.
right dorsolateral prefrontal cortex: division of the prefrontal cortex that organizes withdrawal-related behavior and negative affect and mediates threat-related vigilance. It plays a role in working memory for object location.
rostral: toward the front of the head.
sagittal plane: the plane that divides the body into right and left halves.
saltatory conduction: action potential conduction in myelinated axons in which action potentials jump from node to node for 200 times greater speed.
secondary somatosensory cortex (S2): the region of the parietal lobe adjacent to S1 (BA 40 and 43), receives projections from it and maps touch and pain from both sides of the body.
sensorimotor rhythm (SMR): 13-15 Hz (beta 1) rhythm that is located over the sensorimotor strip (C3, Cz, C4). The waves are synchronous. SMR increases with stillness and decreases with movement. Deficient SMR may be observed in movement spectrum complaints like hyperactivity and tics.
sensorimotor system: in Sterman’s model, ascending pathways that convey information about touch and proprioception to the thalamus, the thalamus and its thalamic projections to the sensorimotor cortex, and the sensorimotor cortex, and its efferent fibers.
sensory event-related potentials (ERPs): event-related potentials evoked by external sensory stimuli (auditory, olfactory, somatosensory, and visual). These evoked potentials or exogenous ERPs have a negative peak around 80-90 ms and a positive peak about 170 ms following stimulus onset. These changes in brain activity in response to specific stimuli. ERPs can be detected throughout the cortex. Investigators monitor ERPs by placing electrodes at locations like the midline (Fz, Cz, and Pz). A computer analyzes a subject's EEG responses to the same stimulus or task over many trials to subtract random EEG activity. ERPs always have the same waveform morphology. Their negative and positive peaks occur at regular intervals following the stimulus.
sensory nerves: neurons specialized for sensory intake. They are called afferent because they transmit sensory information towards the central nervous system.
septal nuclei: a limbic structure that contains several nuclei involved in emotion, control of aggressive behavior, reward, and addiction. The septohippocampal system contributes to the theta rhythm.
septohippocampal system: a subcortical circuit from the septum to the hippocampus that contributes to 4-7 Hz theta activity.
septum: a limbic structure that contains several nuclei involved in emotion and addiction and control of aggressive behavior.
sharp transients: a sequence that contains several sharp waves.
sharp waves: waves that resemble spikes with a pointed peak with a longer 70-200-ms duration.
sink: a site where current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, represented by -ve.
slow cortical potentials (SCPs): gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials. SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms.
sodium (Na+) ions: positive ions that enter a neuron during EPSPs and action potentials.
sodium-potassium transporters: pumps that are powered by ATP and that exchange three sodium for two potassium ions.
soma or cell body: the part of a neuron that contains machinery for cell life processes and receives and integrates EPSPs and IPSPs from axons generated by axosomatic synapses (junctions between axons and somas). The cell body of a typical neuron is 20 μm in diameter, and its spherical nucleus, which contains chromosomes comprised of DNA, is 5-10 μm across.
somatic nervous system: spinal nerves that innervate somatosensory receptors in the skin, joints, and skeletal muscles.
source: the place at the end of the neuron opposite of the sink where current leaves. The source is symbolized by +ve. The extracellular area surrounding the source becomes electrically positive.
spatial summation: the addition of EPSPs and IPSPs over a neuron’s surface.
spike: a negative transient with a pointed peak at conventional paper speeds, 20-70 ms duration, and 40-100 μV amplitude.
spike-and-slow-wave complex: a spike followed by a higher amplitude slow wave at 3 Hz. In an absence seizure, the amplitudes are very high (e.g., 160 μV).
spinal cord: the column of neurons and glial cells within the vertebral canal that extend from the brainstem to the lumbar vertebrae. The spinal cord distributes sensory information from the body to the brain, and CNS commands from the brain to the body. The spinal cord also contains networks that control reflexes and central pattern generators.
spinal nerve: 31 pairs of nerves that exit the spinal cord.
spindle waves: waves that originate in the thalamus and occur during unconsciousness and stage-2 sleep.
striatal: basal ganglia (caudate nucleus and putamen).
stroke: cerebrovascular accident (CVA) involves the destruction of brain tissue (infarction) due to cerebral hemorrhage and cerebral ischemia affecting blood vessels that supply the brain. CVAs show abrupt onset and involve temporary or permanent neurological symptoms like aphasia, paralysis, or loss of sensation.
Stroop test: cognitive monitoring task where color and names conflict.
substantia nigra: the midbrain structure that projects to the basal ganglia (caudate nucleus and putamen) to control movement and is progressively destroyed in Parkinson’s disease.
sulcus: a shallow groove in the surface of the cerebral hemisphere, for example, the central sulcus.
superior colliculus: the dorsal midbrain structure that receives visual information and directs visual gaze and attention to selected stimuli.
superior olivary nuclei: midbrain structures that process binaural information to localize sound.
Sylvian fissure: deep fissure that serves as the upper boundary of the temporal lobe.
synapse-associated polyribosome complexes (SPRCs): organelles with dendrites that can produce proteins that allow rapid remodeling of synapses. A polyribosome complex consists of several ribosomes bound to messenger RNA (mRNA). SPRCs represent one mechanism underlying synaptic plasticity.
synaptic cleft: 20-40 nm fluid-filled gap between presynaptic and postsynaptic structured.
synchronous: adverb meaning that groups of neurons depolarize and hyperpolarize simultaneously.
synchronous "alpha": network-wide "alpha" that integrates perception and facilitates action. This distributed activity appears to block localized alpha-like patterns such as mu and the posterior rhythm in favor of more broadly distributed network integration.
synchrony: the coordinated firing of pools of neurons. EEG signals can display local synchrony, frequency synchrony, and phase synchrony.
telencephalon: the frontal forebrain subdivision that consists of the cerebral cortex, basal ganglia, and limbic system.
temporal summation: the addition of EPSPs and IPSPs over time. Summation is more effective when postsynaptic potentials are generated more closely in time.
temporal lobes: lobes separated from the rest of the cortical lobes by the Sylvian fissure (BA 15, 20, 21, 22, 37, 38, 39, 40, 52). The temporal lobes process hearing, smell, and taste information and help us understand spoken language and recognize visual objects and faces.
terminal buttons: buds located on the ends of axon branches that form synapses and release neurochemicals to other neurons. They contain vesicles that store neurotransmitters for release when an action potential arrives. A terminal button’s presynaptic membrane may have reuptake transporters that return neurotransmitters from the synapse or extracellular space for repackaging.
thalamus: the forebrain structure above the hypothalamus that consists of specialized nuclei that process and relay data to and from the telencephalon (cerebral cortex, basal ganglia, and limbic system). The thalamus analyzes all sensory data except olfaction before distributing this information to the cortex via thalamocortical afferent fibers. The thalamus contributes to SCPs, delta, theta, alpha, SMR activity, and beta-gamma activity.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
threshold of excitation: the membrane potential at which an axon initiates an action potential, nominally -40 mV.
transient: a single wave or sequence of regular waves, called a complex, distinguishable from background EEG activity.
triphasic wave: a wave that contains three deflections from baseline.
unmyelinated axons: smaller-diameter axons without fatty insulation that conduct more slowly than myelinated axons.
+ve: The source is the place at the other end of the neuron where the current leaves. The source is symbolized by +ve.
-ve: A sink is where the current enters the neuron. Positive sodium ion entry into a neuron creates an active sink, symbolized by -ve.
ventral: toward the base of the skull or front of the body.
ventral posterior nucleus (VPN): a thalamic nucleus that receives somatosensory information following crossover at the medulla and projects to the primary somatosensory cortex (S1).
ventral stream (auditory): the subcortical auditory pathway to the auditory cortex that appears to analyze sound components, including speech sounds.
ventral stream (visual): the pathway from the primary visual cortex (V1) to the inferior temporal and frontal areas that allows us to identify objects and faces.
ventral striatum: the olfactory tubercle and nucleus accumbens.
ventral tegmental area: the midbrain structure that distributes dopaminergic axons to the nucleus accumbens. Serotonin receptors on endorphin-releasing neurons in the hypothalamus may increase the activity of dopaminergic reward pathways by inhibiting the release of GABA at receptors on cell bodies of the ventral tegmental area neurons.
ventromedial prefrontal cortex (VMPFC): the ventromedial reward network (BA 10, 14, 25, 32, and parts of 11, 12, and 13) implicated in making decisions where the outcomes are uncertain and where moral values must be applied to actual situations.
vigilance system: in Sterman’s model, a system that consists of both specific brainstem nuclei (e.g., locus coeruleus and raphe nuclei) and their diffuse connections with the thalamus and other subcortical structures, and the cortex. Several neurotransmitter systems mediate vigilance, including cholinergic/glutamatergic (reticular formation), noradrenergic (locus coeruleus), and serotonergic (raphe) neurons.
volume conduction: the movement of the EEG through body tissues and interstitial fluid to the scalp.
volume transmission: extrasynaptic neurotransmitter release from axonal varicosities, dendrites, and terminal button into the extracellular space. Monoamines like norepinephrine and serotonin are released outside the synaptic cleft.
waveform: the shape and form of an EEG signal.
Wernicke's area: area of the temporoparietal cortex (BA 22) of the dominant hemisphere specialized for speech perception and production. Damage can result in an inability to understand the meaning of speech and construct intelligible sentences.
white matter: the layer beneath the cortex that mainly consists of myelinated axons.
REVIEW FLASHCARDS ON QUIZLET
Click on the Quizlet logo to review our chapter flashcards.

Assignment
Now that you have completed this unit, explain the importance of slow cortical potentials. How does 8-10 Hz alpha differ from 10-12 Hz alpha?
References
Advokat, C. D., & Comaty, J. E., & Julien, R. M. (2019). Julien's primer of drug action (15th ed.). Worth Publishers.
Aggleton, J. P., O'Mara, S. M., Vann, S. D., Wright, N. F., Tsanov, M., & Erichsen, J. T. (2010). Hippocampal-anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. Eur J Neurosci, 31(12), 2292-22307. https://doi.org/10.1111/j.1460-9568.2010.07251.x
Aloisi, F. (2001). Immune function of microglia. Glia, 36, 165–179. https://doi.org/10.1002/glia.1106
Aminoff, E. M., Kveraga, K., & Bar, M. (2013), The role of the parahippocampal cortex in cognition. Trends Cogn Sci, 17(8), 379-390. https://doi.org/10.1016/j.tics.2013.06.009
Amzica, F., & Lopes da Silva, F. H. (2018). Cellular substrates of brain rhythms. In Schomer, D. L. & F. H. Lopes da Silva (Eds.). Niedermeyer's electroencephalography: Basic principles, clinical applications, and related fields (7th ed.). Oxford University Press.
Anderson, P. & Anderson, S. A. (1968). Physiological basis of the alpha rhythm. Appleton.
Andreassi, J. L. (2007). Psychophysiology: Human behavior and physiological response (5th ed.). Lawrence Erlbaum and Associates, Inc.
Arnsten, A. F. (2006). Fundamentals of Attention-Deficit/Hyperactivity Disorder: Circuits and pathways. Journal of Clinical Psychiatry, 67 (Suppl. 8), 7-12.
Babiloni, C., Babiloni, F., Carducci, F., Cincotti, F., Del Percio, C., Hallett, M., Moretti, D. V., Romani, G. L., & Rossini, P. M. High resolution EEG of sensorimotor brain functions: Mapping ERPs or mu ERD? In R. C. Reisin, M. R. Nuwer, M. Hallett, & C. Medina (Eds.). Advances in Clinical Neurophysiology (Supplements to Clinical Neurophysiology Vol. 54). Elsevier Science B. V.
Bastos, A. M., & Schoffelen, J-M. (2016). A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front Syst Neurosci. https://doi.org/10.3389/fnsys.2015.00175
Bear, M. F., Connors, B. W., & Paradiso, M. A. (2020). Neuroscience: Exploring the brain (4th ed.). Jones & Bartlett Learning.
Beauregard, M., & Levesque, J. (2006). Functional magnetic resonance imaging investigation of the effects of neurofeedback training on the neural bases of selective attention and response inhibition in children with Attention-Deficit/Hyperactivity Disorder. Applied Psychophysiology and Biofeedback, 31(1), 3-20. https://doi.org/10.1007/s10484-006-9001-y
Breedlove, S. M., & Watson, N. V. (2020). Behavioral neuroscience (9th ed.). Sinauer Associates, Inc.
Cameron, H. A., & Dayer, A. G. (2008). New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry, 63(7), 650-655. https://dx.doi.org/10.1016%2Fj.biopsych.2007.09.023
Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature, 10, 186-198. https://doi.org/10.1038/nrn2575
Caplan, D. (2006). Why is Broca’s area involved in syntax? Cortex, 42, 469–471. https://doi.org/10.1016/S0010-9452(08)70379-4
Carlson, N. R., & Birkett, M. A. (2017). Physiology of behavior (12th ed.). Pearson Education, Inc.
Catmur, C., Walsh, V., & Heyes, C. (2007). Sensorimotor learning configures the human mirror system. Cur Biol, 17(17), 1527-1531. https://doi.org/10.1016/j.cub.2007.08.006
Chan, C. Y., Ke, D. S., & Chen, J. Y. (2009). Essential fatty acids and human brain. Acta Neurol Taiwan, 18(4), 231-241. PMID: 20329590
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Costanzo, R. M. (1991). Regeneration of olfactory receptor cells. CIBA Found Symp, 160, 233-242.
Creuzfeldt, O. D. (1995). Cortex cerebri. Oxford University Press.
Dahl, M. J., Mather, M., Sander, M. C., & Werkle-Bergner, M. (2020). Noradrenergic responsiveness supports selective attention across the adult lifespan. J Neurosci, 40(22), 4372-4390. https://doi.org/10.1016/j.tics.2021.10.009
Dahl, M. J., Mather, M., & Werkle-Bergner, M. (2022). Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends in Cognitive Sciences, 26(1), 38-52. https://doi.org/10.1016/j.tics.2021.10.009
Damasio, A. (2010). Self comes to mind. Pantheon Books.
deCharms, R. C., Fumiko, M., Glover, G. H., Ludlow, D., Pauly, J. M., Soneji, D., Gabrieli, J. D. E., & Mackey, S. C. (2005). Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences, 102(51), 18626-18631.
DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci, 13(7), 281-285. https://doi.org/10.1016/0166-2236(90)90110-v
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Diamond, A. (2013). Executive functions. Annu Rev Psychol, 64, 135-168. https://doi.org/10.1146/annurev-psych-113011-143750
Dyro, F. M. (1989). The EEG handbook. Little, Brown and Company.
Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302, 290-292. https://doi.org/10.1126/science.1089134
El-Boustani, S., Ip, J., Breton-Provencher, V., Knott, G., Okuno, H., Bito, H., & Sur, M. (2018). Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science, 360(6395), 1349-1354. https://doi.org/10.1126/science.aao0862
Enticott, P. G., Kennedy, H. A., Rinehart, N. J., Tonge, B. J., Bradshaw, J. L., Taffe, J. R., Daskalakis, Z. J., & Fitzgerald, P. B. (2012). Mirror neuron activity associated with social Impairments but not age in Autism Spectrum Disorder. Biol Psychiatry, 71(5), 427-433. https://doi.org/10.1016/j.biopsych.2011.09.001
Evans, J. R., & Abarbanel, A. (1999). Introduction to quantitative EEG and neurofeedback. San Diego: Academic Press.
Farwell, L. A., & Donchin, E. (1991). The truth will out: Interrogative polygraphy (“lie detection”) with event-related brain potentials. Psychophysiology, 28, 531–547. https://doi.org/10.1111/j.1469-8986.1991.tb01990.x
Fisch, B. J. (1999). Fisch and Spehlmann's EEG primer: Basic principles of digital and analog EEG (3rd ed.). Elsevier.
Fischer, D. B., Boes, A. D., Geerling, J. C., Edlow, B., Pascula-Leone, A., & Fox. M. (2016). The neuroanatomic basis of coma in humans: A study of brainstem lesions and their cortical networks (S52.003). Neurology, 84(14), Supplement S52.003. https://dx.doi.org/10.1212%2FWNL.0000000000003404
Fuster, J. (2015). The prefrontal cortex (5th ed.). Academic Press.
Garrett, B. (2003). Brain and behavior. Thompson/Wadsworth.
Hale, J. B., & Fiorello, C. A. (2004). School neuropsychology: A practitioner's handbook. Guilford Press.
Hansson, E., & Ronnback, L. (2003.) Glial neuronal signaling in the central nervous system. FASEB J, 17, 341-348. https://doi.org/10.1096/fj.02-0429rev
Herbet, G., & Duffau, H. (2020). Revisiting the functional anatomy of the human brain: Toward a meta-networking theory of cerebral functions. Physiol Rev, 100, 1181-1128. https://doi.org/10.1152/physrev.00033.2019
Hindriks, R., & van Putten, M. J. A. M. (2013). Thalamo-cortical mechanisms underlying changes in amplitude and frequency of human alpha oscillations. NeuroImage, 70, 150-163. https://doi.org/10.1016/j.neuroimage.2012.12.018
Hugdahl, K. (1995). Psychophysiology: The mind-body perspective. Harvard University Press.
Hughes, S. W., & Crunelli, V. (2005). Thalamic mechanisms of EEG alpha rhythms and their pathological implications. The Neuroscientist, 11(4), 357-372. https://doi.org/10.1177/1073858405277450
Hughes S. W., & Crunelli, V. (2007). Just a phase they're going through: The complex interaction of intrinsic high-threshold bursting and gap junctions in the generation of thalamic alpha and theta rhythms. Int J Psychophysiol, 64(1), 3-17. https://doi.org/10.1016/j.ijpsycho.2006.08.004
Izhikevich, E. M., & Edelman, G. M. (2008). Large-scale model of mammalian thalamocrotical systems. Proceedings of the National Academy of Sciences of the United States of America, 105(9), 3593-3598. https://doi.org/10.1073/pnas.0712231105
Kalat, J. W. (2019). Biological psychology (13th ed.). Cengage Learning.
Kandel, E., Koester, J. D., Mack, S. H., & Siegelbaum, S. (2021). Principles of neural science (6th ed.). McGraw-Hill Education.
Kennerley, S. W., Behrens, T. E., & Wallis, J. D. (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci, 14(12), 1581-1589. https://doi.org/10.1038/nn.2961
Kitamura, T., Saitoh, Y., Takashima, N., Murayama, A., Niibori, A., Ageta, H., . . . Inokuchi, K. (2009). Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell, 139(4), 814-827. https://doi.org/10.1016/j.cell.2009.10.020
Kleen, J. K., Chung, J. E., Sellers, K. K., Zhou, J., Triplett, M., Lee, K., Tooker, A., Haque, R., & Chang, E. F. (2021). Bidirectional propagation of low frequency oscillations over the human hippocampal surface. Nature Communications, 12(2764). https://doi.org/10.1038/s41467-021-22850-5
Klein, S. B., & Thorne, B. M. (2007). Biological psychology. Worth Publishers.
Krauss, G. L., Fischer, R. S., & Kaplan, P. W. (Eds.) (2011). The John Hopkins atlas of digital EEG: An interactive training guide. The John Hopkins University Press.
Kropotov, J. D. (2009). Quantitative EEG, event-related potentials and neurotherapy. Academic Press.
Kringelbach, M. L. (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci, 6(9), 691-702. https://doi.org/10.1038/nrn1747
Landisman, C. E., & Connors, B. W. (2005). Long-term modulation of electrical synapses in the mammalian thalamus. Science, 310(5755), 1809-1813. https://doi.org/10.1126/science.1114655
Lubar, J. F. (1997). Neocortical dynamics: Implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Applied Psychophysiology and Biofeedback, 22(2), 111-126. https://doi.org/10.1023/a:1026276228832.
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct, 214(5-6), 655-667. https://dx.doi.org/10.1007%2Fs00429-010-0262-0
Meshorer et al. (2002). Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science, 295(5554), 508-512. https://doi.org/10.1126/science.1066752
Miller, B. L., & Cummings, J. L. (Eds.) (2007). The human frontal lobes: Functions and disorders (2nd ed.). Guilford Press.
Molenberghs, P., Cunnington, R., & Mattingley, J. B. (2011). Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev, 36(1), 341-349. https://doi.org/10.1016/j.neubiorev.2011.07.004
Molle, M., Marshall, L., Gais, S., & Born, J. (2002). Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. The Journal of Neuroscience, 22, 10941-10947. https://doi.org/10.1523/JNEUROSCI.22-24-10941.2002
Monastra, V. J., Lubar, J. F., & Linden, M, VanDeusen, P., Green, G., Wing, W., . . Fenger, T.N. (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validiation study. (2001). Neuropsychology, 13(3), 424-433. https://doi.org/10.1037/0894-4105.13.3.424
Monti, J. M., & Jantos, H. (2008). The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res, 172, 625-646. https://doi.org/10.1016/S0079-6123(08)00929-1
Munro, C. A., McCaul, M. E., Wong, D. F., Oswald, L. M., Zhou, Y., Brasic, J., Kuwabara, H., Kumar, A., Alexander, M., Ye, W., & Wand, G. S. (2006). Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry, 59(10), 966-974. https://doi.org/10.1016/j.biopsych.2006.01.008
Nash, J. M. (2011). The gift of mimicry. Your brain: A user's guide. Time.
Neumann, N., Strehl, U., & Birbaumer, N. (2003). A primer of electroencephalographic instrumentation. In M. Schwartz & F. Andrasik (Eds.). Biofeedback: A practitioner's guide (3rd ed.). Guilford Press.
Ongür, D., & Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex, 10(3), 206-219. https://doi.org/10.1093/cercor/10.3.206
Radley, J. J., Arias, C. M., & Sawchenko, P. E. (2006). Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. The Journal of Neuroscience, 26(50), 12967-12976. https://doi.org/10.1523/JNEUROSCI.4297-06.2006
Raichle, M. E., & Gusnard, D. A. (2002). Appraising the brain's energy budget. PNAS, 99(16), 10237-10299. https://doi.org/www.pnas.orgcgidoi10.1073pnas.172399499
Rajmohan, V., & Mohandas, E. (2007). Mirror neuron system. Indian J Psychiatry, 49(1), 66-69. https://doi.org/10.4103/0019-5545.31522
Ramachandran, V. S. (2011). The tell-tale brain: A neuroscientist's quest for what makes us human. New York: W. W. Norton & Company.
Rapanelli, M., Frick, L. R., & Zanutto, B. S. (2011). Learning an operant conditioning task differentially induces gliogenesis in the medial prefrontal cortex and neurogenesis in the hippocampus. PLoS ONE, 6(2), e14713. doi:10.1371/journal.pone.0014713
Rizzolatti, G., & Sinigaglia, C. (2008). Mirrors in the brain: How our mind share actions, emotions, and experience. Oxford University Press.
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L., & von Stein, A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci, 95(12), 7092-7096. https://doi.org/10.1073/pnas.95.12.7092
Schacter, D. L. (1977). EEG theta waves and psychological phenomena: A review and analysis. Biological Psychology, 5, 47-82. https://doi.org/10.1016/0301-0511(77)90028-x
Schomer, D. L., & Lopes da Silva, F. H. (2011). Niedermeyer's electroencephalography: Basic principles, clinical applications, and related fields (6th ed.). Lippincott Williams & Wilkins.
Stahl, S. M. (2008). Stahl’s essential psychopharmacology: Neuroscientific basis and practical applications (3rd ed.). Cambridge University Press.
Steriade, M. (2001). The intact and sliced brain. MIT.
Steriade, M. (2005). Cellular substrates of brain rhythms. In E. Niedermeyer, & F. Lopes da Silva (Eds.). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
Steriade, M., & Llinás (1988). The functional states of the thalamus and the associated neuronal interplay. Physiol Rev, 68(3), 649-742. https://doi.org/10.1152/physrev.1988.68.3.649
Sterman, M. B. (2000). EEG markers for attention deficit disorder: Pharmacological and neurofeedback applications. Child Study Journal, 30(1), 1-24.
Sterman, M. B., & Kaiser, D. A., (2001). Comodulation: A new QEEG analysis metric for assessment of structural and functional disorders of the CNS. Journal of Neurotherapy, 4(3), 73-83. https://doi.org/10.1300/J184v04n03_05
Stern, R. M., Ray, W. J., & Quigley, K. S. (2001). Psychophysiological recording (2nd ed.). Oxford University Press.
Streit, W. J. (2006). Microglial senescence: Does the brain's immune system have an expiration date? Trends in Neurosciences, 29(9), 506–510. https://doi.org/10.1016/j.tins.2006.07.001
Thatcher, R. W., Biver, C. J., & North, D. M. (2009). EEG and brain connectivity: A tutorial. Unpublished manuscript.
Thompson, M., & Thompson, L. (2009). Asperger’s syndrome intervention: Combining neurofeedback, biofeedback, and metacognition. In T. H. Budzynski, H. K. Budzynski, J. R. Evans, & A. Abarbanel (Eds.). Introduction to quantitative EEG and neurofeedback (2nd ed.). Academic Press.
Thompson, M., & Thompson, L. (2015). The biofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Applied Psychophysiology and Biofeedback.
Traub, R. D., Miles, R., & Wong, R. K. S. (1989). Model of the origin of rhythmic population oscillations in the hippocampal slice. Science, 243, 1319-1325. https://doi.org/10.1126/science.2646715
van den Heuvel, M. P., & Sporns, O. (2011). Rich-club organization of the human connectome. J Neurosci, 31(44), 15775-15786. https://doi.org/10.1523/JNEUROSCI.3539-11.2011
Voloh, B., & Womelsdorf, T. (2016). A role of phase-resetting in coordinating large scale neural networks during attention and goal-directed behavior. Front Syst Neurosci, 10, doi:10.3389/fnsys.2016.00018
Voytek, B. (2013). Brain metrics: How measuring brain biology can explain the phenomena of mind. Scitable by Nature Education.
Warren, A. M., & McIlvane, W. J. (1998). Stimulus equivalence and the N400 effect. Poster presented at the 1998 Annual Meeting of the Cognitive Neuroscience Society in San Francisco, CA.
Wilson, J. (2003). Biological foundations of human behavior. Wadsworth/Thompson Learning.
Wilson, V. E., Thompson, M., Thompson, L., Thompson, J., Fallahpour, K., & Linden, M. K. (2011). Introduction to biofeedback (Neurofeedback). In B. W. Strack, M. K. Linden, & V. S. Wilson (Eds.). Biofeedback & neurofeedback applications in sport psychology. Association for Applied Psychophysiology and Biofeedback.
Winn, P. (2001). (Ed.), Dictionary of biological psychology. Routledge.
Xu, T., Yu, X., Perlik, A., Tobin, W., Zweig, J., Tennant, K., Jones, T., & Zuo, Y. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature, 462(7275), 915-919
Yang, Y., Ge, W., Chen, Y., Zhang, Z., Shen, W., Wu, C., Poo, M., & Duan, S. (2003). Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proceedings of the National Academy of Sciences of the United States of America, 100(25), 15194-15199. https://doi.org/10.1073/pnas.2431073100
Zaid, D. H., & Andreotti, C. (2010). Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia, 48(12), 3377-3391. https://doi.org/10.1016/j.neuropsychologia.2010.08.012
Zhang, H., Watrous, A., Patel, A., & Jacobs, J. (2018). Theta and alpha oscillations are traveling waves in the human neocortex. Neuron. https://doi.org/10.1016/j.neuron.2018.05.019
TEST YOURSELF ON CLASSMARKER
Click on the ClassMarker logo below to take a 10-question exam over this entire unit.

